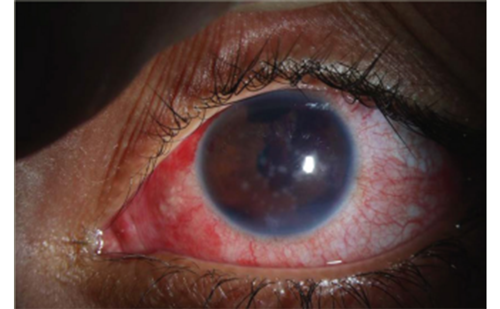
The accumulation of transudative fluid in the suprachoroidal space, known as choroidal effusion, is a prevalent and often asymptomatic complication of glaucoma surgery. Choroidal effusions have also been linked with other aetiologies, including infections, inflammatory diseases, medications and other ocular surgeries.1,2 While choroidal effusions do not typically lead to long-term complications, if particularly large or persistent, they can precipitate more serious sequelae, including suprachoroidal haemorrhage, aqueous hyposecretion and anterior chamber shallowing, leading to increased risk of vision loss.3 While small choroidal effusions will frequently resolve spontaneously with time and standard postoperative medications, more persistent effusions may require more aggressive medical or even surgical interventions. This article focuses on the recent literature regarding medical and surgical management of choroidal effusions, with attention to choroidal effusions that develop postoperatively following traditional glaucoma surgery.
Overview of choroidal effusions
The physiology of choroidal effusions is driven by the hydrostatic and osmotic forces among the suprachoroidal space and choriocapillaris. Intraocular pressure (IOP) drives the hydrostatic pressure gradient of the suprachoroidal space, while the osmotic pressure is generated by protein and fluid diffusion through the highly permeable choriocapillaris.4 In the setting of ocular hypotony driving a decrease in interstitial hydrostatic pressure and leading to a higher capillary pressure relative to interstitial pressure, along with the presence of a large inflammatory response and subsequent protein and fluid leakage from the choriocapillaris, a choroidal effusion can develop in the suprachoroidal space, particularly following glaucoma surgery.5 Of note, many patients may have marked hypotony without the subsequent development of choroidal effusions, likely due to the lack of inflammation and leakage of protein from the choroidal vessels.4 Due to the contribution of both hypotony and pro-inflammatory conditions on choroidal effusion development, ocular surgery (glaucoma surgery, in particular) can predispose a patient to choroidal effusions in the postoperative period. Procedures that lead to more substantial IOP reductions, such as implantation of glaucoma drainage devices (GDD) or trabeculectomy, typically have higher incidences of postoperative choroidal effusions. Past studies and reviews have reported postoperative choroidal effusion incidence rates ranging from 3.0% to 18.8% following trabeculectomy, 13% to 15% following Ahmed™ Glaucoma Valve Model FP7 (AGV-FP7) (New World Medical, Rancho Cucamonga, CA, USA) placement, and 11.7% to 14.0% following Baerveldt glaucoma implant placement (Johnson & Johnson Surgical Vision, Inc., Irvine, CA, USA).3,6 For procedures that are less invasive, the rates of choroidal effusions have been proposed to be slightly lower, potentially due to the lower likelihood of very low postoperative IOPs. The incidences reported in the literature for microinvasive glaucoma surgeries (MIGS) vary depending on the specific type of MIGS performed. For instance, the InnFocus MicroShunt Versus Trabeculectomy Study (ClinicalTrials.gov Identifier: NCT01881425) found a postoperative choroidal effusion incidence rate of 4.6% in the MicroShunt group as compared to 6.1% in the trabeculectomy group.7 However, this difference was not significant.7 The rate of choroidal effusions following insertion of the XEN gel stent (Allergan, an AbbVie company, North Chicago, IL, USA) has also been reported, with ranges between 1.3% and 19.8% depending on the technique implemented.8–12 While serous choroidal effusions occur due to the accumulation of transudative fluid in the suprachoroidal space, a haemorrhagic choroidal detachment is defined by the accumulation of blood in this space due to the rupture of posterior ciliary artery branches. Haemorrhagic choroidal detachments can also occur due to drops in IOP leading to arterial rupture or inflammation both in the intraoperative and postoperative periods. Haemorrhagic choroidal detachments that occur in the intraoperative period are ocular emergencies often requiring surgical intervention.
Beyond glaucoma surgery, other aetiologies linked to the formation of choroidal effusions include the usage of certain medications and preexisting patient conditions. Medications such as sulfonamides, anticonvulsants, bisphosphonates, select monoclonal antibody medications and tetracyclines have been reported to be associated with choroidal effusion development.13–16 In terms of preexisting conditions, various factors including older age, systemic hypertension, hyperopia, Sturge-Weber Syndrome, pseudoexfoliative glaucoma, pseudophakia and increased central corneal thickness have been reported to be associated with increased risks of choroidal effusion development.17–21 In patients with Sturge-Weber Syndrome in particular, choroidal effusions that form following filtration surgery have been found to be larger, with more rapid onset and higher prevalence.22
Medical management of choroidal effusions
Generally, small choroidal effusions are managed conservatively and tend to resolve over time and with standard postoperative medications.23 As IOP rises in the postoperative period, most effusions will resolve on their own if they are smaller in size and do not affect visual, ocular, or surgical prognoses. Following glaucoma surgery, leakage from surgical wounds (due to an epithelialized fistula track) may also contribute to the generation of choroidal effusions.24,25 In these cases, resolution of the effusion may be achieved by ensuring adequate closure of surgical wounds. In cases where observation alone is insufficient, medical management of choroidal effusions involves topical corticosteroids to decrease inflammation and topical cycloplegic eye drops to rotate the lens–iris diaphragm posteriorly.26–28 In cases that do not resolve with topical agents, systemic corticosteroids may also be used.
Various approaches to topical and systemic therapies have been reported in the literature. For instance, while the standard-of-care therapy with systemic steroids typically involves a regimen of a low-to-moderate dose of oral corticosteroids in the postoperative period, a case report published by Kanamoto and Takamatsu commented on the potential success of systemic steroid-pulse therapy.29 They report the case of a patient with pseudoexfoliation glaucoma whose choroidal effusions following cataract surgery and glaucoma filtration surgery did not respond to a 20-day course of prednisolone 20 mg/day. A regimen of methylprednisolone sodium succinate 1,000 mg/day for 3 days was then utilized, with a subsequently observed increase in IOP and choroidal effusion resolution.29 The use of sub-tenon triamcinolone acetonide (TA) injections has also been reported in the literature. A prospective case series found that a sub-tenon TA injection led to resolution of serous choroidal effusions in 16 patients whose choroidal effusions were non-resolving after use of systemic steroids, topical atropine and topical steroids following glaucoma surgery.30
Procedural approaches are also available to manage choroidal effusions. These approaches typically target postoperative hypotony, especially following glaucoma surgery, with the injection of a cohesive viscoelastic agent such as 1% sodium hyaluronate or alternative agents (e.g., 100% sulphur hexafluoride gas [SF6]) in the anterior chamber (AC) to deepen the AC and increase IOP.31
Overall, it is likely that a majority of choroidal effusions, particularly those following glaucoma surgeries, resolve with conservative management alone. In a case report of eight cases of choroidal effusions following trabeculectomies, all patients in the report achieved improvement or complete resolution after medical treatment with topical cycloplegic and corticosteroid agents and oral corticosteroids within 3–4 weeks of treatment.32 In a retrospective study by the authors of this review article investigating the postoperative course of 110 patients who developed choroidal effusions following GDD surgery, 83% of patients had resolution of choroidal effusions without requiring more invasive surgical interventions. In the study, 48% of patients achieved choroidal effusion resolution via medical management alone, while 35% of patients achieved effusion resolution following one or more anterior chamber viscoelastic injections.33
While medical and procedural management of choroidal effusions are the first-line approaches to this postoperative complication, investigation into different methods to mitigate postoperative hypotony, particularly through viscoelastic fill of the anterior chamber at AGV-FP7 the time of surgery, have been reported in the literature with mixed results. A study by Gulkilik et al. prospectively analyzed the use of a sodium hyaluronate injection into the anterior chamber after suturing of the scleral flap during trabeculectomy.34 Compared with a group that received a balanced salt solution injection, the study group receiving the sodium hyaluronate injection had significantly higher mean IOPs on postoperative day 1 and significantly lower occurrences of hypotony, anterior chamber shallowing and development of choroidal effusions.34 However, a study by Dugan et al. described the use of an intracameral injection of sodium hyaluronate 1% before conjunctival peritomy at the beginning of AGV-FP7 implantation to achieve an IOP of ~20 mmHg by finger tension.35 However, unlike previous studies, there was no significant difference in early postoperative complication rates including hypotony, AC shallowing and choroidal effusions between patients who received and those who did not receive intraoperative viscoelastic fill.35 Further investigation into the use and effects of intraoperative AC viscoelastic injections is needed to understand its effects in the postoperative period.
As with all medical and procedural approaches, potential complications should be taken into account when deciding whether or not to proceed with a particular management approach in a patient. While AC viscoelastic injection is considered to be a relatively safe approach to choroidal effusion management, the type and timing of viscoelastic device utilized should take into account a patient’s individual surgical scenario and clinical profile. Particularly with the increased applications of innovative devices within glaucoma surgery such as MIBS (microinvasive bleb surgery), the use of select viscoelastic devices should be approached with caution. Bruynseels et al. reported a case of a patient with primary open angle glaucoma who experienced hypotony to an IOP of 2 mmHg, a shallow AC and choroidal effusions following insertion of a XEN gel stent. Healon 5 (0.1 mL) (Johnson & Johnson Surgical Vision, Inc., Irvine, CA, USA), a pseudodispersive ultraviscous viscoelastic device was injected into the AC, and 12 hours following injection, the patient experienced significant pain and reduced visual acuity in the eye with an IOP of 70 mmHg. The AC was seen to have complete viscoelastic device fill, with no visible cellular movement. Due to the viscoadaptive properties of Healon 5 and the low flow rate of the XEN gel stent (2–3 μL/min), Healon 5 acts as an ultraviscous cohesive agent at low flow rates and would act as a virtual solid in these patients. The authors cautioned against Healon 5 use for postoperative hypotony after XEN gel stent insertion to avoid potentially sight-threatening stent occlusion and IOP spikes.36
In terms of the approach utilized by the authors of the current review, several techniques are employed to minimize postoperative hypotony risk in the postoperative period following glaucoma surgeries. In the case of GDDs, for implantation of non-valved devices, a temporary ligature is released in the office at postoperative weeks 4–5 via green argon or diode laser to disrupt the polyglactin suture. The anterior chamber is then filled with a cohesive viscoelastic. Aqueous suppressants are usually stopped in the week leading up to the planned ligature release. For patients receiving Ahmed devices, the anterior chamber is always filled with viscoelastic at the end of the surgery, and if the IOP falls below 6 mmHg within the first postoperative week, additional viscoelastic is typically injected into the anterior chamber.
Surgical management of choroidal effusions
For patients whose choroidal effusions are refractory to medical or procedural management, surgical interventions can be considered. There are several indications for surgical drainage of choroidal effusions including a flat anterior chamber with lens-cornea apposition, persistent corneal oedema with a flat anterior chamber, prolonged effusion duration, appositional choroidal effusion, severe pain, elevated IOP despite medical therapy, and any concern for vision loss.2,3 Typically, the need for surgical intervention in managing choroidal effusions in the postoperative period is relatively infrequent. A study by Chu et al., found that 3 out of 662 patients (0.4%) who underwent a wide array of glaucoma procedures (e.g., trabeculectomy, GDD, cytophotocoagulation, trabectome) required surgery for choroidal effusion resolution within a 90-day postoperative period.27 Other studies that focused on glaucoma procedures with higher risks of postoperative hypotony and choroidal effusions reported slightly higher figures. The Tube Versus Trabeculectomy study (ClinicalTrials.gov Identifier: NCT00306852) saw that 2% of a patient cohort who underwent GDD placement required surgical drainage of postoperative choroidal effusions, and 1% of patients who underwent trabeculectomy required surgical drainage.37 When looking specifically at a cohort of patients who presented with choroidal effusions following GDD placement, a retrospective study performed by the authors of this review found that 17% required surgical intervention to drain the effusion or ligate the implant tube.
Surgical drainage of choroidal effusions is generally regarded as a relatively safe and successful procedure, with reported success rates of around 77% in achieving effusion resolution and visual acuity improvement.38 A study by WuDunn et al. reported that in patients who underwent one or more drainage procedures for choroidal effusions, IOP at 6 and 12 months following drainage was significantly higher compared to pre-drainage IOP.38 Similarly, visual acuity measurements at 6- and 12-months post-drainage were significantly improved compared to the pre-drainage period. However, as with any surgical intervention, drainage of choroidal effusions and return to the operating room carry increased risks of complications compared to more conservative approaches to management, including infection, uncontrolled IOP and recurrence of effusions. A retrospective study performed by the authors of this review found that a higher percentage of patients who required multiple surgical interventions for effusion resolution lost two or more lines of Snellen Visual Acuity at the most recent follow-up compared to patients whose effusions resolved with medical or procedural management.33
With the generally increased risks of surgical interventions for choroidal effusion management, recent studies have described innovative and alternative methods for the drainage of suprachoroidal fluids through less invasive manners.39,40 Further investigation is needed to fully understand the impacts, benefits and risks of more novel approaches to choroidal effusion drainage.
Of note, the majority of studies included in this article studied choroidal effusions in the adult patient population. Additional investigation into choroidal effusions and their presentation in the pediatric population can help expand the understanding of how to manage this complication in other patient groups.
Conclusion
Choroidal effusions are a common complication of glaucoma surgery and manifestation of other etiologies including certain medications and preexisting conditions. Most choroidal effusions can be managed with conservative management including topical and systemic medications, as well as procedural approaches such as anterior chamber viscoelastic fill. Surgical intervention may be necessary for patients with particularly large or persistent effusions, and further investigation into the various methods of choroidal effusion management is needed to achieve optimal patient and postoperative outcomes.