Despite the introduction of several new microinvasive glaucoma operations in recent years, traditional glaucoma surgeries – trabeculectomy and glaucoma drainage device implantation (GDI) – remain commonly performed incisional procedures for the management of various types of glaucoma.1,2 This article focuses on the medical and surgical management of blebs following traditional glaucoma surgery – trabeculectomy and tube shunt surgery.
Basics of trabeculectomy
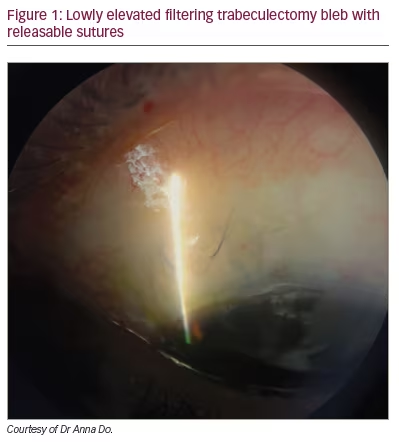
Trabeculectomy with antifibrotic adjuvant therapy, such as mitomycin-C (MMC) and 5-fluorouracil (5-FU), uses the anti-scarring effects of the medication to improve the success rates of filtration surgery.3 MMC acts by alkylating guanine bases, while 5-FU inhibits thymidylate synthase; both agents ultimately prevent fibroblast- and lymphocyte-mediated fibrosis.4 Intraoperative antifibrotics can be administered via a subconjunctival injection or applied with saturated sponges on the scleral bed after a conjunctival flap is dissected. In elderly patients with thin conjunctiva, some surgeons opt for postoperative 5-FU injections to mitigate the risk of hypotony and bleb leak. A scleral flap is created and closed with 10-0 nylon sutures after making a sclerostomy and iridectomy. Most glaucoma specialists choose to place at least 2–4 sutures through the scleral flap to allow titration of flow postoperatively by sequential laser suture lysis or pulling releasable sutures as needed. A tight trabeculectomy flap with minimal early flow allows the conjunctiva wound to heal and is less prone to early leaks. An elevated intraocular pressure (IOP) in the early postoperative period is more easily remedied than hypotony from an overfiltering trabeculectomy flap. (Figure 1 shows a low filtering bleb with two releasable sutures.)
Trabeculectomy complications and how to manage them
Early bleb leak
Bleb leaks within the first month of surgery are most commonly due to inadequate conjunctival wound closure. The Tube Versus Trabeculectomy (TVT) study detected a wound leak in 12 of 105 cases (11%) within the first month following trabeculectomy.5 Though a bleb/wound leak can result in numerous complications, the most concerning issue arising from an early leak is early failure of the trabeculectomy. Without elevation of the bleb, there is often accelerated episcleral fibrosis in the area of the scleral flap. In most cases of an early leak (more commonly seen with a fornix-based conjunctival flap), a simple way to encourage healing is to apply a large-diameter bandage contact lens. Another conservative option is to prescribe gentamicin or tobramycin ointment, which irritates the conjunctival epithelium and stimulates scarring. Aqueous suppression with beta blockers, a2-adrenergic agonists or carbonic anhydrase inhibitors may aid in healing by slowing the flow of aqueous. Cyanoacrylate glue, fibrin tissue glue and autologous blood injection have also been successfully used in limited numbers of patients, though the latter may be better suited for a late leak.6 Re-suturing at the slit lamp can be effective for smaller leaks but is challenging to perform. Larger leaks, on the other hand, typically require revision in the operating room. Meticulous wound closure at the time of initial closure with a tight scleral flap offers the best protection against an early leak. Ensuring the conjunctiva is well approximated and that the leading edge is not ‘rolled inwards’ is important, as this will promote optimal healing.
Late bleb leak
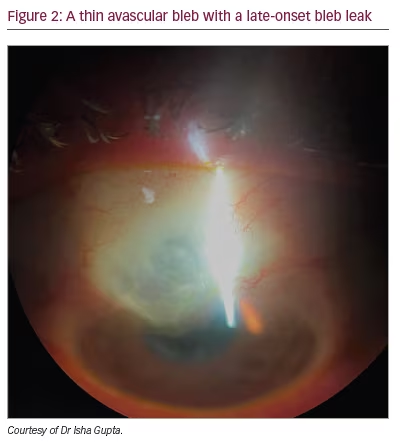
Bleb leaks that occur months to years after surgery are typically found on routine examination of the bleb, as patients are often asymptomatic initially. Studies have shown a higher risk of blebitis and endophthalmitis with a late-onset bleb leak.7 Hypotony-related complications are also more prevalent with late leaks. Since the introduction of antimetabolites in glaucoma filtering surgery, the incidence of spontaneous bleb leaks has increased to 1.8–10.0% after 5 years.8 Greenfield et al. found that intraoperative MMC leads to a higher incidence of post-operative bleb leak compare to 5-FU (3.7% versus 1.4%).9 Late bleb leaks are more difficult to treat, as the leak usually occurs in areas of extremely thin and avascular conjunctiva under greater tension, as shown in Figure 2. In these cases, the gold standard is revision of the surgical site with conjunctival advancement or a rotational conjunctival flap. The revision should aim to undermine the tissue as much as possible in order to recruit sufficient healthy conjunctiva to cover the avascular area without tension. Care must be taken to de-epithelialize the roof of the existing bleb prior to advancing and securing healthy conjunctiva to the limbus. Relaxing incisions or rotational flaps, and placement of amniotic membrane may aid in covering large defects. In these cases, patients must be counselled that there is a risk for increased fibrosis and potential bleb failure. Another helpful technique for late leaks at the bleb apex is to needle the bleb and encourage more posterior flow. This helps to flatten the bleb profile and relieve the tension in the area of the leak.10
Fibrosis
Scarring at the episcleral surface or flap interface can lead to a flat bleb and elevated IOP. A failing bleb can be salvaged but requires prompt recognition and early treatment with 5-FU (or MMC depending on the timing of failure), and possible needle revision at the slit lamp or in the operating room. Skuta et al. compared intraoperative MMC with postoperative 5-FU and found that, at 6 months after surgery, eyes that had received intraoperative MMC had lower overall IOP, lower glaucoma medication usage and decreased corneal toxicity.11 The timing of intervention for a bleb that still has flow is crucial, as the onset of effect for 5-FU is delayed. The appearance of the bleb rather than the IOP should dictate when and how often the medication is administered. 5-FU at 5 or 10 mg can be injected adjacent to the filtering site and care should be taken to prevent excess medication from refluxing out through the needle track, as this can result in corneal toxicity. For patients who present with elevated IOP and a flat-appearing bleb, revision is best performed in the operating room, as the scleral flap needs to be elevated and additional MMC placed. The authors’ preference is to only revise when the edge of the flap is visible beneath the conjunctiva, as this offers the best chance for successful revision. In situations where this is not the case, placement of a glaucoma drainage device may be a more suitable option.
Bleb encapsulation occurs when an elevated bleb appears tight, thick and vascular. This can be treated conservatively with aqueous suppression to reduce the surface tension on the bleb wall before considering bleb needling with antimetabolites or surgical intervention.12 Encapsulation of the filtering bleb tends to occur with greater frequency following tube shunt surgery but can present following trabeculectomy and even after placement of newer microshunt devices, such as the Xen gel stent (Allergan Inc., North Chicago, IL, USA) and PreserFlo MicroShunt (Santen, Osaka, Japan).
Bleb dysesthesia
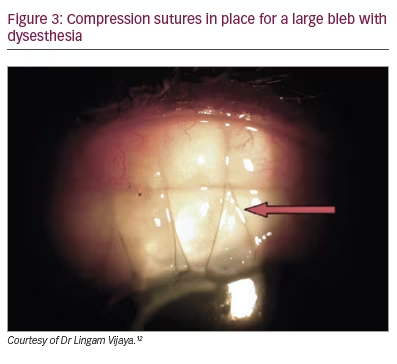
Large or overhanging blebs can cause symptoms associated with the resulting tear-film abnormalities, dellen formation and ocular surface irregularities. When frequent lubrication fails to resolve a patient’s bleb dysesthesia, revision surgery should be considered to reduce the height or size of the bleb.7 The Palmberg compression mattress suture is one technique that involves passing sutures to compress and reduce the bleb’s fluid conductivity, which ultimately leads to remodelling of the bleb. This has been shown to be 80% successful in resolving bleb dysesthesia in 46 eyes.13 The sutures (9-0 or 10-0 nylon) are anchored in the corneal limbus, passed posteriorly over the portion of bleb to be compressed, and secured with an episcleral bite, then passed back anteriorly over the bleb in a shoelace or mattress pattern (Figure 3). Multiple sutures can be placed with varying tightness depending on the surgeon’s desire to compress the bleb in each area or to remove sequentially as needed to titrate the compression effect. Polyglactin sutures can also be used to induce conjunctival inflammation and fibrosis of the bleb in specific areas if desired.
Basics of glaucoma drainage implantation
Tube shunts provide a conduit for aqueous to drain from within the eye to a subconjunctival bleb, further posteriorly than a trabeculectomy bleb, over the reservoir endplate. There are two types of tube shunts: valved and non-valved glaucoma drainage implants.
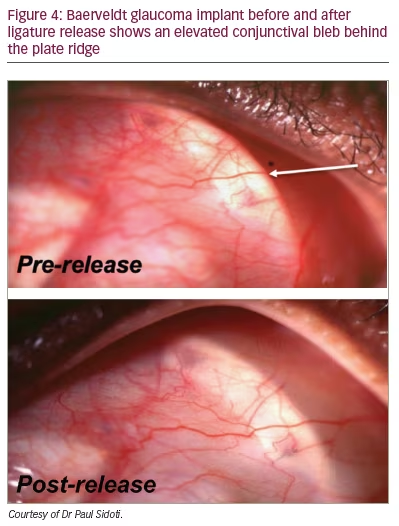
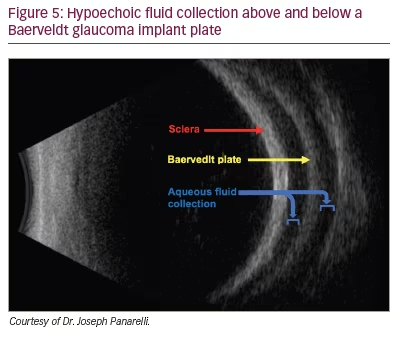
For typical non-valved glaucoma drainage implants (Baerveldt glaucoma implant – Johnson & Johnson, Santa Ana, CA, USA; or Molteno implant – Nova Eye Medical, Fremont, CA, USA and Dunedin, New Zealand), the tube is ligated with a 7-0 absorbable polyglactin suture to allow the conjunctiva to heal and the endplate to encapsulate. Early flow can be established during this time using a 10-0 polyglactin or nylon suture wick and/or fenestrations through the tube lumen. If the IOP is elevated in the early postoperative period, glaucoma medications with relatively short half-lives should be used and aqueous suppressants should be minimized if possible so that aqueous production is normal when the tube opens. When the ligature releases, there is usually a decrease in IOP. Other signs that the tube has opened include mild anterior chamber cell and fibrin, an elevated conjunctival bleb over the reservoir plate (Figure 4) and a hypoechoic fluid collection overlying the plate on B-scan ultrasound (Figure 5).
Valve devices such as the Ahmed Glaucoma Implant (New World Medical, Rancho Cucamonga, CA, USA), on the other hand, allow for immediate flow and do not require tube ligation, as the flow-restricting mechanism helps protect against overfiltration in the early postoperative periods.
Hypertensive phase
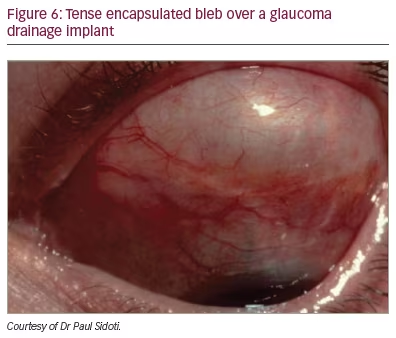
Encapsulation around the endplate occurs over the first 2–6 weeks following implantation.14 Factors that contribute to bleb formation and encapsulation include patient age, race, preoperative IOP, cytokine content and individual reaction to the presence of a foreign body in the subconjunctival space.15 A ‘hypertensive phase’ occurs when there is an elevation in IOP, usually with a well-circumscribed capsule and vascular congestion around the GDI endplate (Figure 6). Though the exact mechanism of the hypertensive phase is unclear, it is hypothesized that early exposure to inflammatory factors in aqueous humour can lead to dense fibrous encapsulation.16 Others have speculated that prolonged elevated IOP within the bleb results in cytokine production by the bleb lining, causing decreased permeability of the fibrous tissue of the capsule wall. Anticipating a hypertensive phase is important in the monitoring of patients postoperatively, as it has been shown that early and aggressive medical management (when IOP is still in the low teens) helps to decrease the incidence and magnitude of the hypertensive phase.17 Though the hypertensive phase is typically noted after Ahmed valve implantation, it will be seen with similar frequency following implantation of non-valved devices and similar management should be undertaken.
Histopathological studies suggest that early aqueous drainage results in overall thicker capsule walls (average 475 μm), with a more robust initial fibrovascular reaction compared with capsules from staged procedures with initial tube occlusion, which resulted in thinner capsule walls (average 220 μm).18 Pooled data from the Ahmed Baerveldt comparison study and the Ahmed versus Baerveldt study both demonstrate this concept in their findings: Ahmed implantation produced a greater IOP reduction immediately postoperatively, but Baerveldt implantation tended to result in a lower IOP and a lower 5-year failure rate.19
Intraoperative and postoperative use of antifibrotics has been studied in Ahmed valve implantation. Perez et al. retrospectively evaluated 17 eyes undergoing Ahmed valve surgery with serial subconjunctival MMC injections administered intraoperatively, postoperatively at Week 1 and postoperatively at Week 4, compared with 20 eyes undergoing Ahmed valve surgery without MMC. Those that received MMC required fewer glaucoma medications at 6 months and had a lower incidence of hypertensive phase (17.6%) compared with the eyes that did not receive MMC (55.0%).20 However, adjuvant MMC use in Ahmed valve surgery has also been shown to increase the risk of hypotony (31.81% with MMC vs 15.38% without MMC) and late tube erosion (13.63% with MMC vs 0% without MMC).21
Conclusion
Tube shunt surgery and trabeculectomy remain a mainstay in the glaucoma surgeon’s armamentarium. Careful surgical technique, close postoperative monitoring, and anticipatory and aggressive management of hypertensive phases can help patients achieve safe outcomes and long-term IOP control.







