The challenge of patient adherence
Patient adherence is a major concern in the field of glaucoma, where medication adherence rates can be as low as 40–55% due to complex dosing regimens, unpleasant side effects and difficulty with eyedrop administration.1,2 Moreover, 25% of patients with glaucoma do not refill their second eyedrop prescription, making it challenging for physicians to preserve patient vision and ensure treatment compliance.3 The use of topical medications can also lead to ocular surface disease and interfere with wound healing, adding to the problems faced by patients with glaucoma.4 The importance of reducing the antiglaucoma eyedrop burden has been established by several studies, and this has led to development of novel sustained-release platforms for glaucoma medications.5–9 These innovations could potentially improve patient adherence, whilst also reducing potential side effects. Several extraocular and intraocular platforms, which are in advanced stages of development, will offer welcome alternatives for glaucoma care providers.
Therapeutic options in development
Extraocular therapeutic options include:
-
wearable ocular surface devices (such as gel-forming eyedrops, topical ocular drug delivery devices, drug-impregnated contact lenses, ocular insert rings and collagen shields)
-
punctal plug systems (such as the travoprost ophthalmic insert [OTX-TP; Ocular Therapeutix, Bedford, MA, USA] and the latanoprost punctal plug delivery system)
-
subconjunctival injections (such as the subconjunctival latanoprost [Eye-D VS-101; BioLight Life Sciences, Tel Aviv, Israel; IBI-60089; Icon Bioscience, Sunnyvale, CA, USA], latanoprost solution [POLAT-001; Peregrine Ophthalmic, Punggol BayView, Singapore], prodrug [GB-6249-103; Graybug Vision Inc, Baltimore, MD, USA], and dorzolamide-loaded polymer microparticles).5
Intraocular platforms include intracameral implants such as:
-
the bimatoprost sustained-release (Durysta®; Allergan, Irvine, CA, USA; AbbVie Inc, North Chicago, IL, USA)
-
travoprost extended release (XR) (ENV515; Envisia Therapeutics, Morrisville, NC, USA)
-
travoprost implant (iDose® TR; Glaukos, San Clemente, CA, USA)
-
travoprost intracameral implant (OTX-TIC; Ocular Therapeutix, Bedford, MA, USA)
-
latanoprost free acid sustained release ocular implant (PA5108; PolyActiva, Melbourne, Victoria, Australia)
-
omidenepag isopropyl (OMDI) (DE117; Developed by Santen Pharmaceutical Co., Ltd, Osaka, Japan)-loaded poly (L-lactide-ran-ε-caprolactone) copolymers5,10,11
This editorial will focus on the current status of the iDose TR sustained-release travoprost implant in glaucoma treatment, which has recently reported results from the phase IIb exchange trial, and from phase III clinical trials.12–14
iDose TR travoprost implant
The iDose TR is a titanium travoprost-containing implant, measuring 1.8 mm × 0.5 mm, placed in the anterior chamber via a small corneal incision and held there by a scleral anchor (Figure 1).15 The implant is covered with a membrane containing 78.0 µm of travoprost, which elutes continuously at therapeutic levels for long periods of time.15 There are two versions of the device (slow- and fast-release), which differ in their rate of drug elution.15 The surgical procedure involves creating a 2.2 mm incision and filling the anterior chamber with a cohesive viscoelastic.16 The nasal angle is visualized using a gonioprism, in the same manner as other minimally invasive glaucoma surgery (MIGS) procedures.16 The iDose TR loader is aimed at the trabecular meshwork. Direct perpendicular pressure is applied to anchor the iDose TR implant into the scleral wall and the prongs are released. The loader is used to nudge the device from side to side, to make sure the device is away from the iris. This is followed by irrigation and aspiration to remove the viscoelastic, and ports are hydrated with balanced salt solution.16 Recent data indicates that the device can be safely exchanged for a new device after 4–5 years.12
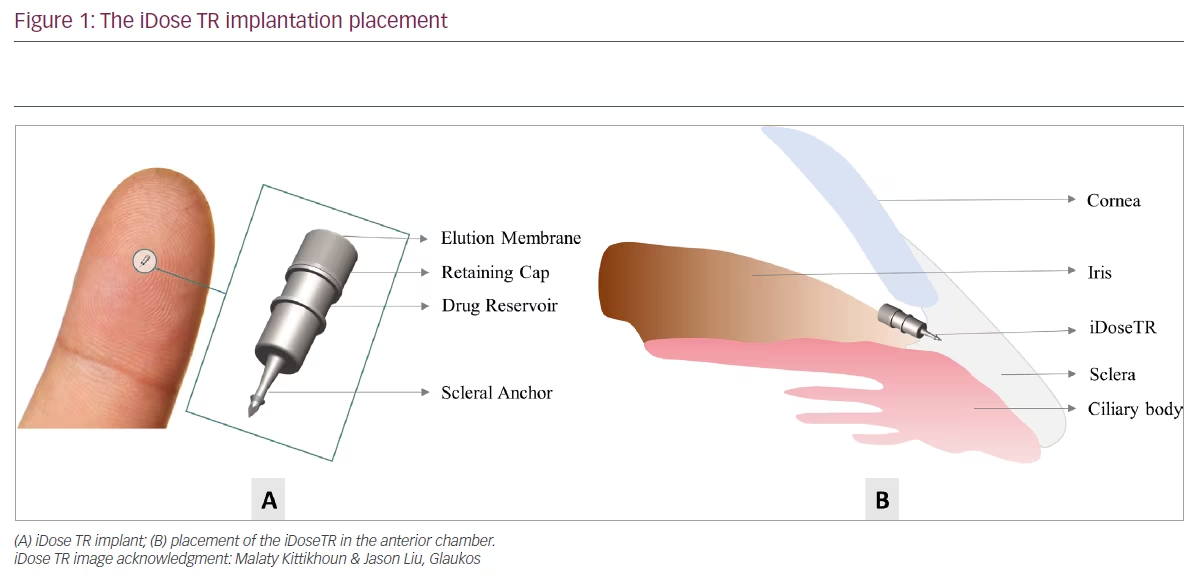
Current evidence
Phase II trials
A summary of clinical trials evaluating the iDose TR in patients with glaucoma can be found in Table 1.12–14,17–22 The GC-009, phase II, multicentre, randomized, double-blind trial, enrolled 154 patients (ClinicalTrial.gov Identifier: NCT02754596).17–19 The patients were randomly assigned 51:54:49 to the fast-release implant, slow-release implant, or sham surgery followed by topical timolol ophthalmic solution, 0.5% (administered twice a day) over a 36-month evaluation period. All three groups had unmedicated baseline intraocular pressures (IOPs) of approximately 25.0 mmHg. The primary endpoint was IOP reduction at 12 weeks post implantation, with secondary endpoints being IOP at 12 months, avoidance of additional topical antiglaucoma drugs at 12 weeks, and adverse effect profile. At the primary endpoint of 12 months, the fast-release implant lowered IOP by 8.5 mmHg (33% reduction from baseline), the slow-release implant lowered IOP by 8.0 mmHg (32% reduction from baseline), and timolol lowered IOP by 7.6 mmHg (30% reduction from baseline).23 There were no serious adverse events. The phase IIb results showed that 70%, 68% and 46% of participants in the fast-release and slow-release iDose TR arms, respectively, were well controlled with the same or fewer IOP-lowering topical medications at 36 months versus screening.18 This is compared with 46% of subjects who were well controlled in the timolol arm.
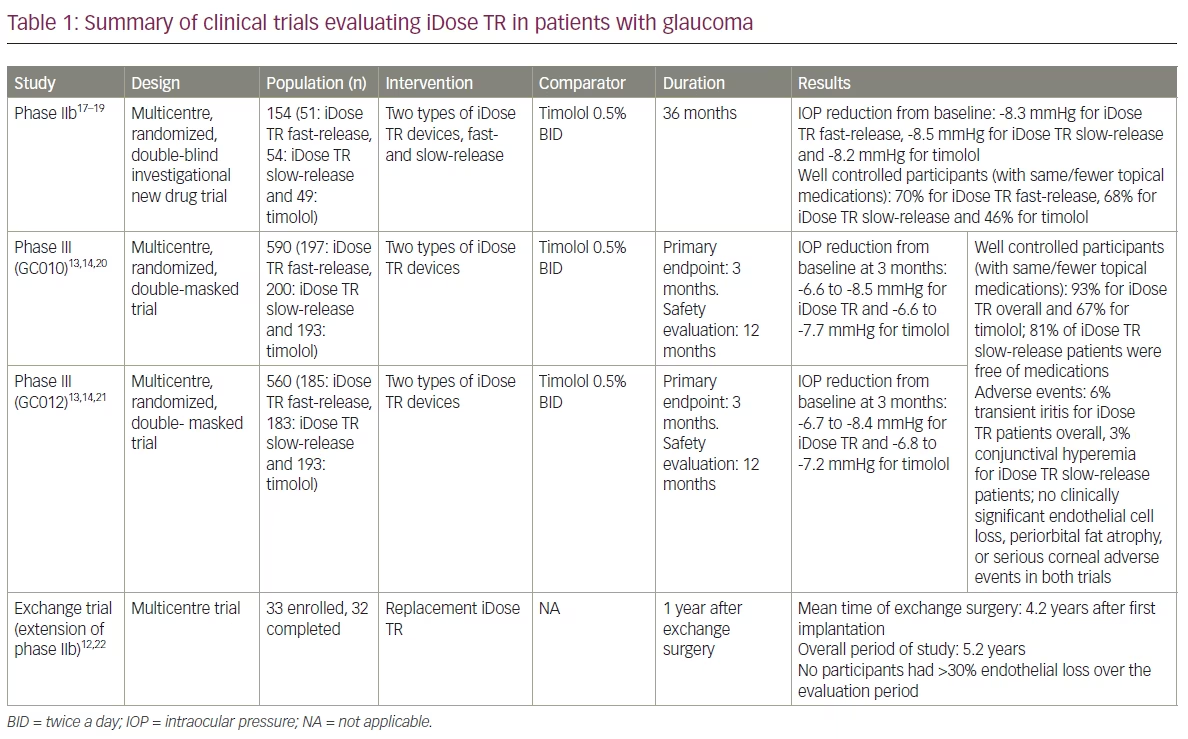
Phase III trials
The phase III clinical programme for iDose TR implant comprised two clinical trials that were randomized, double-masked, and prospective in nature. The two phase III trials randomized a total of 1,150 participants across 89 clinical sites in the USA (ClinicalTrials.gov Identifiers: NCT03519386 and NCT03868124).13,14,20,21 The main aim of these trials was to evaluate the safety and effectiveness of one of the two iDose TR implant models, with varying rates of travoprost release (GC-010 for slow and GC-012 for fast implant), in comparison with a topical timolol ophthalmic solution, 0.5% (administered twice a day), for reducing elevated IOP in individuals suffering from ocular hypertension or open-angle glaucoma. The two trials were almost identical in terms of their protocol, design, size, predefined endpoints and randomization. In both the phase III iDose TR pivotal trials, both implant arms (fast- and slow-release), achieved the prespecified primary efficacy endpoint of non-inferiority to the active comparator arm (topical timolol) through 3 months.13 Most (93%) slow-release participants had well controlled IOP with the same or fewer antiglaucoma topical medications at 12 months versus screening after a single iDose TR implant administration, compared with 67% of participants receiving timolol in both trials. Nearly 81% of slow-release implant participants were free of the topical antiglaucoma medications at 12 months.13 The implants also demonstrated good safety profiles, with no corneal surface events or endothelial cell loss. There were also no events of periorbital fat atrophy. Conjunctival hyperaemia was seen in 3% of participants who received slow-release iDose TR, and the most frequent adverse event was mild transient iritis at a rate of 6% in both phase III trials. As a result of these promising results, Glaukos submitted a new drug application (NDA) to the United States Food and Drug Administration (FDA) for the slow-release iDose TR model.24 The outcome of the FDA’s review is expected by the end of 2023, and iDose TR could be available for use by 2024–25.12,25
Exchange trial
The iDose TR implant is designed to be replaced with a new implant after the drug has been fully administered. In the exchange procedure, the surgeon inserts the new implant and anchors it into the sclera, at least 30° away from the previous implant. The previous implant is removed using an empty inserter. In the exchange trial, 33 participants from the previous phase IIb clinical trial were enrolled, and 32 participants (97%) completed the trial.12 The average time between the administration of the first iDose TR implant (first implantation cycle) in the phase IIb trial and the exchange procedure of the second iDose TR implant (second implantation cycle) in the exchange trial was 4.2 years. The exchange trial participants were then followed for an additional year, resulting in an average overall extended evaluation period of 5.2 years. The results from the exchange trial indicated that removing the original iDose TR implant and administering a second implant was safe and well tolerated. The second implant also demonstrated a favourable safety profile over the 12-month follow-up period. None of the participants in the trial exhibited more than 30% endothelial cell loss over the evaluation period.12 Whilst a small amount of endothelial loss would not be detrimental in younger patients, such a loss can become problematic in older patients with a limited endothelial reserve, who are at risk of corneal decompensation. Once all trial results are formally published, average endothelial loss and trend of loss over time can provide clarity regarding the safety threshold of the implant. Moreover, it is also unclear how clinicians will determine the appropriate time to perform the implant exchange. Several other real-world issues, such as whether individual patient characteristics (e.g. lens status, angle anatomy or implant location) will affect implant performance over time will also need due consideration.
What does this mean for clinical practice?
The salient advantage of the iDose TR implant is the possibility of offering long-lasting eyedrop-free therapeutic options to patients with glaucoma. This can significantly improve quality of life and preserve vision in these patients. It could also reduce long-term treatment costs, provided that the pricing of the implant is not prohibitive, especially for low- and middle-income countries.
Clinicians also need to consider the commercially available bimatoprost intracameral implant (Durysta®; Allergan, Irvine, CA, USA; AbbVie Inc, North Chicago, IL, USA), which has demonstrated a comparable IOP reduction of 5–8 mmHg from baseline over a 20-month period.26,27 A higher percentage of patients reported conjunctival hyperaemia (27%) with this implant, compared with results reported by the iDose TR (3%).14,26 However, direct comparisons between the devices cannot be made and will require more clinical data. However, as the insertion procedure of the implants is invasive, implantation can be considered alongside a combined cataract and MIGS procedure. Implantation can also be done alongside standalone MIGS to keep the patient eyedrop-free for a longer period, however efficient patient counselling will be required to encourage patients to opt for a surgical procedure for IOP control. The implants may also be used alongside a non-valved glaucoma drainage device, to control IOP until the tube of the drainage device opens up. Implantation as a standalone device may also be considered for patients with mild-to-moderate glaucoma. It is also imperative to understand that currently only manufacturer trials are available. Once the implants become commercially available, further long-term results will shed light on the sustainability of using drug-eluting implants in glaucoma management.







