Choroidal granuloma is a relatively rare manifestation of sarcoidosis. Cases of sarcoid granuloma may lack accompanying ocular inflammation and can mimic posterior segment tumours, leading to a delay in diagnosis and treatment. These lesions are typically steroid responsive,1 and quick identification and treatment can result in improved visual outcomes.2 We report two cases of patients who presented with choroidal granulomas of unknown cause, later diagnosed as sarcoidosis. Both responded initially to systemic corticosteroids, then worsened after drug cessation. Both had different clinical courses and were eventually maintained on steroid-sparing immunosuppressive agents.
Results
Informed consent was obtained from both patients described herein.
Case 1
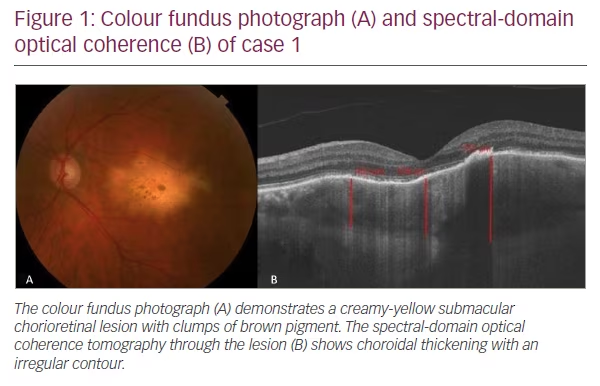
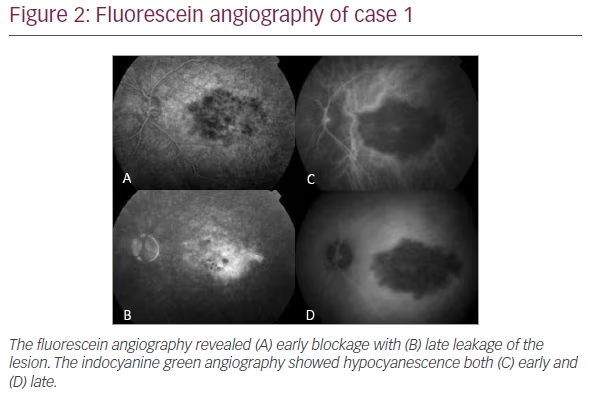
A 60-year-old man presented with vision loss in his left eye (OS) for 1 month. Visual acuity at presentation was 20/20 in the right eye (OD) and 20/40 in the OS. Anterior segment examination of both eyes was unremarkable, whilst posterior segment evaluation revealed a solitary submacular creamy-yellow lesion in the OS (Figure 1A). Spectral-domain optical coherence tomography (OCT) revealed massive choroidal thickening with overlying subretinal fluid and retinal pigment epithelial (RPE) changes. Outer retinal changes were also evident (Figure 1B). Fluorescein angiography revealed early blockage (Figure 2A) with late staining (Figure 2B), while indocyanine green angiography revealed hypocyanescence both early (Figure 2C) and late (Figure 2D). Initial laboratory workup was negative for syphilis (assessed by fluorescent treponemal antibody absorption test (FTA-ABS) and syphilis immunoglobulin G), angiotensin-converting enzyme, antinuclear antibody, lysozyme, Lyme titer and tuberculosis. Chest x-ray and gallium scan were normal. The patient was healthy with a normal review of systems.
A presumptive diagnosis of focal choroiditis was made. The lesion initially responded well to the systemic corticosteroids, and visual acuity remained stable at 20/40 at 3 months. During the corticosteroid course, the patient developed insomnia and mood changes, so the medication was rapidly tapered without progression of the lesion.
The patient noted an acute change in vision 1 year after presentation. On examination, the visual acuity was 20/100, and the lesion had grown with several satellite lesions. The patient refused to go back on oral steroids due to the intolerable side effects. At this time, the decision was made to pursue a tissue diagnosis, so he underwent a choroidal biopsy. The biopsy was performed during 25-gauge pars plana vitrectomy with a 27-gauge needle that was passed through the temporal macula area where the choroidal lesion was thickest. No endolaser was applied, and an air-fluid exchange was performed. The results of the biopsy demonstrated macrophages, pigment and foci of calcification consistent with but not diagnostic of sarcoidosis. He agreed to an injection of intravitreal dexamethasone implant, which led to an improvement in the lesion at 6 weeks post-injection. At 2 months post-injection, he noticed a curtain in his vision and, on examination, was found to have a macula-off retinal detachment. This was repaired by pars plana vitrectomy and instillation of perfluoropropane gas. At 3 months after his initial retinal detachment surgery, he developed a recurrent retinal detachment and required another surgery. At the preoperative physical exam in preparation for this surgery, his heart rate was 40; an electrocardiogram revealed a 3rd-degree heart block (complete heart block).
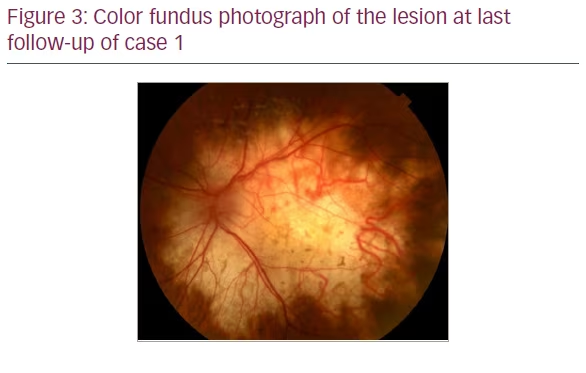
The patient was urgently admitted to the cardiac intensive care unit, where he was diagnosed with cardiac sarcoidosis by computerized tomography (CT) of the chest. A cardiac pacemaker was implanted. He was treated with a short course of systemic corticosteroids, which was bridged to steroid-sparing immunosuppressive therapy with mycophenolate mofetil. When systemically stable, he underwent retinal detachment repair by pars plana vitrectomy and instillation of 1,000-centistoke silicone oil. His cataract was also removed during the surgery. At 4 years after the diagnosis of sarcoidosis and initiation of mycophenolate, the patient’s lesion remained stable, and there were no other systemic complications related to sarcoid (Figure 3). His visual acuity was counting fingers at 3 feet, and his retina was attached.
Case 2
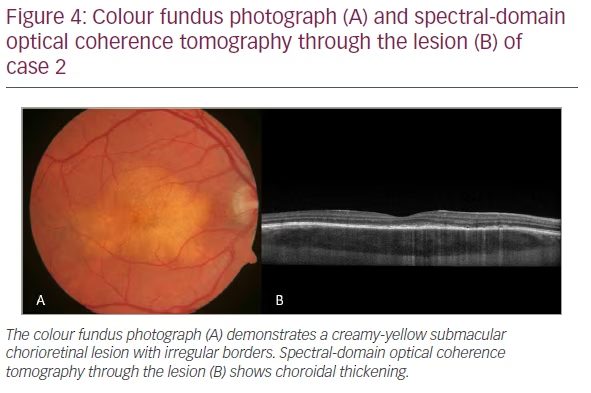
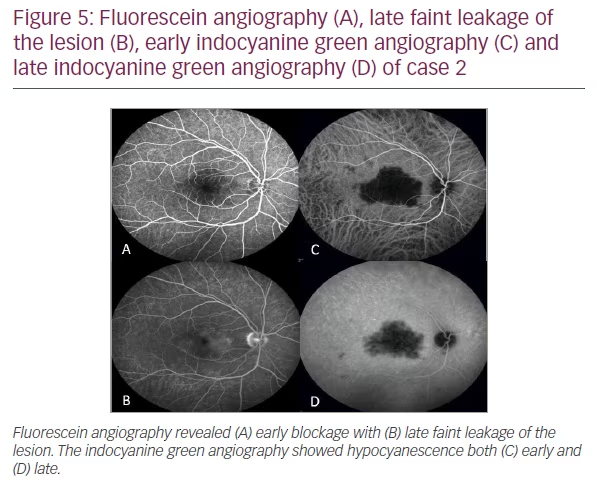
A 35-year-old man presented with distorted vision in the OD for 1 month following an upper respiratory illness. Visual acuity was 20/20 in both eyes. Anterior segment examination was unremarkable. Posterior segment evaluation in the OD revealed a submacular choroidal lesion with irregular borders (Figure 4A). There was no evidence of inflammation. OCT revealed choroidal thickening with overlying RPE and outer retinal disruption and mild subretinal fluid (Figure 4B). Fluorescein angiography was unrevealing at 1 minute (Figure 5A) but showed late staining of the choroidal lesion at 13 min (Figure 5B). There was hypocyanescence in the early and late frames of indocyanine green angiography (Figures 5C and D). Angiotensin-converting enzyme, lysozyme, rapid plasma reagin (RPR), fluorescent treponemal antibody absorption, and Quantiferon tests were negative. Chest x-ray and chest CT were normal. The patient was healthy with no medical problems and a normal review of systems.
Due to the clinical suspicion of sarcoidosis, the patient was started on oral prednisone and monitored with serial examinations. His symptoms and exam improved after a short course of oral prednisone but worsened when the steroids were tapered. A repeat chest CT was performed and showed pulmonary nodules. A bronchoscopy of his perihilar lymph nodes confirmed the diagnosis of sarcoidosis. He restarted oral prednisone and was bridged to methotrexate. On subsequent visits, he was noted to have decreased choroidal thickening and resolution of the RPE and outer retinal changes. At 6 years from diagnosis, his choroidal granuloma remained stable on oral methotrexate with 20/20 vision. He has remained healthy without any other manifestations of sarcoid.
Discussion
Sarcoidosis is a systemic inflammatory disease that is characterized by the formation of non-caseating granulomas. It can affect many organ systems, including the heart, lungs, skin and lymph nodes. Ocular sarcoidosis has a variety of presentations. The most common ocular manifestation is anterior uveitis, which occurs in about 60% of patients with ocular involvement.3 Posterior involvement is less common, with choroidal involvement only described in 5% of patients with ocular sarcoidosis.4 Diagnosis of choroidal sarcoid granuloma can be challenging. Unlike anterior uveitis related to sarcoidosis, choroidal sarcoid granulomas often exhibit little to no inflammation in the eye, which contributes to delayed presentation.5,6 In addition, solitary choroidal sarcoid granulomas can mimic other posterior segment lesions, such as choroidal osteoma or metastatic carcinoma, making diagnosis difficult.5
Choroidal granulomas from sarcoidosis often present as pale yellow, well-defined choroidal lesions with irregular borders. Choroidal granulomas are vision threatening if located in or near the macula, as the retina above the lesion can be affected by serous neurosensory detachment and, eventually, atrophy. OCT can be key to diagnosing the condition, with enhanced depth imaging demonstrating homogenous shadowing in the choroid.7–9 If ocular sarcoidosis is suspected, a full systemic workup should be initiated, including pulmonary scans to identify any hilar adenopathy or other signs of pulmonary sarcoidosis. Tissue diagnosis can be critical for the accurate diagnosis and management of sarcoidosis. In one case series, diagnostic vitrectomy provided a diagnosis of sarcoidosis in 42% of patients, suggesting that this method of diagnosis is supportive but not definitive in cases of presumed sarcoidosis-related posterior inflammation.10
Despite initially having similar appearing choroidal lesions, the two patients described had different clinical courses. The first patient in this case report developed cardiac involvement, a complication of sarcoidosis that can be fatal. Cardiac involvement in sarcoidosis is uncommon, affecting 6% of all patients.11 Sarcoid granulomas in the heart can cause conduction abnormalities and myocardial wall changes, leading to arrhythmia, atrioventricular block and cardiomyopathy. Due to high clinical suspicion and low threshold to biopsy, the second patient in this case report avoided systemic complications from sarcoidosis with early steroid-sparing immunosuppression.
Corticosteroids are the mainstay of treatment for ocular and systemic sarcoidosis. Choroidal granulomas from sarcoidosis are often steroid responsive, with patients exhibiting improvement or resolution of symptoms and ocular findings after initiation of treatment.2 Local steroid therapies, such as injections into the sub-tenon’s space12 or vitreous cavity,13,14 have also been used with symptomatic improvement. Furthermore, steroid-sparing immunosuppressive agents are effective in treating ocular and systemic sarcoidosis. Both patients described responded well to oral steroids but worsened when they were discontinued. Prompt referral to a rheumatologist for consideration of steroid-sparing immunosuppression is recommended when sarcoidosis is suspected.
Conclusion
Choroidal granulomas from sarcoidosis are a rare but important condition that must be diagnosed and treated promptly. Delayed diagnosis and treatment can result in worsened visual outcomes and systemic complications, which can be fatal. Ophthalmologists should be aware of this condition and maintain a high level of suspicion, as patients can present with ocular symptoms prior to systemic involvement.







