Retinal pharmacotherapy encompasses various drug delivery routes that offer potential avenues for effective treatment (Figure 1). Among these, intravitreal injections have emerged as the predominant method employed in clinical practice.1 They have established themselves as the primary approach for administering anti-vascular endothelial growth factor (anti-VEGF) therapy, serving as the frontline treatment for neovascular age-related macular degeneration (AMD), diabetic macular oedema (DMO), and macular oedema secondary to retinal vein occlusion (RVO) among other less frequent pathologies in which similar treatments are used. Intravitreal steroid injections also provide adjunctive or alternative treatment in DMO, RVO, and uveitis. Recently, there has been clinical interest in exploring the suprachoroidal space (SCS) as a potential route for retinal drug delivery. This anatomical region, characterized by a gap length of approximately 35 µm, delineates the area between the choroid and the overlying sclera.2 In a manner similar to intravitreal injections, suprachoroidal injections are amenable to in-office procedures and offer distinct advantages by facilitating direct drug delivery to the retina, choroid and retinal pigment epithelium (RPE). Moreover, suprachoroidal delivery holds promise for mitigating drug exposure to the anterior segment, thereby curtailing the accompanying side effects commonly associated with drug exposure to anterior ocular structures.3
The purpose of this review article is to revisit the fundamentals and recent utilizations of suprachoroidal delivery. Special attention is given to its more recent application to axitinib therapy, as seen in the OASIS trial (ClinicalTrials.gov identifier: NCT04626128), CLS-301 (Clearside Biomedical, Inc., Alpharetta, GA, USA), as well as other small molecules, that have been under investigation for therapeutic potential.
Figure 1: Retinal injection delivery routes
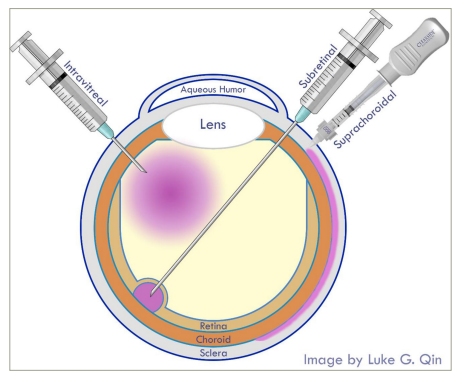
Image was customly generated by the author using Adobe Photoshop.
Flow mechanics of suprachoroidal delivery
The posterior segment of the eye poses challenges for drug delivery, due to both static and dynamic barriers. Static barriers include the sclera, choroid, Bruch’s membrane and the blood–retinal barrier (BRB). The sclera is composed of irregularly arranged collagen fibrils, serving as a significant obstacle to the passage of foreign substances into the posterior ocular tissues.4 Subsequently, lipophilic drugs and large molecules face limited permeability through the aqueous scleral pores.4,5 The choroid, a vascular layer, contributes to drug equilibration and elimination, before compounds can reach Bruch’s membrane.5 Accumulated cell debris at Bruch’s membrane further hampers the exchange of nutrients and drugs.6 The posterior segment is additionally fortified by the BRB, featuring tight junctions between the inner retinal vascular endothelium and outer RPE.7 Suprachoroidal drug delivery offers a solution to circumvent these barriers and limitations, enabling more effective and targeted administration of therapeutic agents to the posterior segment of the eye. Various techniques have been developed for this purpose, including manual injections with a syringe and needle, as well as use of the SCS Microinjector® (Clearside Biomedical, Inc., Alpharetta, GA, USA).8 This device is equipped with a microneedle measuring either 900 µm or 1,100 µm in length, precisely calibrated to reliably access the SCS in an office setting.9 Suprachoroidal injections are generally well tolerated, with a low incidence of serious adverse events.10 Common side effects include transient ocular discomfort and mild intraocular pressure elevation, but severe complications such as suprachoroidal haemorrhage, retinal detachment, endophthalmitis and other emergent adverse events are rare.8
Understanding flow mechanics of the SCS is fundamental for optimizing drug distribution and efficacy (Figure 2).11 Initial investigations involved suprachoroidal injection of polymer into rabbit eyes.12 These investigations demonstrated that suprachoroidal injection of the polymer was not only safe and well tolerated but was also a reliable and nontraumatic method of drug delivery. Histological analyses revealed minor changes, including the formation of vacuoles, with no sign of inflammation in the eye tissue. Additionally, fluorescein angiography confirmed that the presence of the polymer had no adverse effects on the retinal and choroidal vasculature. The ability of the polymer to persist in the SCS suggests its potential for sustained drug delivery in therapeutic applications. Complementing this, brightfield/fluorescence microscopy, as well as microcomputed tomography have been employed to comprehensively characterize the flow mechanics within the SCS.13 These simulations provided insights into flow patterns and drug distribution within the SCS. Notably, in contrast to molecules, which underwent relatively quick clearance from the SCS, polymeric particles exhibited chorioretinal selectivity and remarkable persistence within this environment for months.
Figure 2: Flow mechanics and drug distribution of suprachoroidal injection11
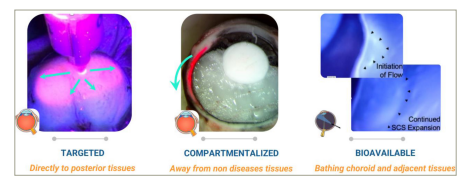
From left to right: fluoresceine dye under UV light shows posterior and circumferential spread of injectate, frozen dye injectate shows compartmentalized distribution and reduced exposure to anterior chamber and vitreous body, and ex vivo endoscopic visualization of suprachoroidal injection in enucleated porcine eyes using an aqueous-based green dye revealed smooth internal deformation and immediate posterior and circumferential flow of injectate upon accessing the SCS.
Image reused with permission from Clearside Biomedical, Inc.
SCS = suprachoroidal space.
Comparing suprachoroidal route’s flow mechanics to that of the intravitreal route has been of recent interest too. The potential for faster clearance of fluid and drugs from the eye via the suprachoroidal route as supposed to intravitreal route has been reported, suggesting lower outflow resistance.13 Fluorophotometry has been used to demonstrate that slow decline in drug levels with the intravitreal route can be attributed to the gradual absorption of the drug from the vitreous humor into the choroid, whereas if in the choroid, the drug is seen to rapidly clear from the tissue due to the denser vascularization and higher flow rate in this region.14 The efficient clearance of the drug from the choroid is attributed to its robust blood perfusion.
The phase I/II HULK trial (ClinicalTrials.gov identifier: NCT02949024) shows the anatomical changes in the SCS following suprachoroidal injection of triamcinolone acetonide (CLS-TA) for DMO.15 An immediate, but transient opening of the SCS after injections is seen, with no significant short-term changes the SCS compared to fellow eyes.15 However, in the TANZANITE trial (ClinicalTrials.gov identifier: NCT02303184), the suprachoroidal administration of CLS-TA for macular oedema secondary to RVO revealed significant short-term expansion of the SCS, but the space would return to normal within approximately one month’s time.16 Data on the long-term anatomical and physiological changes in the SCS following drug delivery remain limited and speculative.
Multimodal imaging techniques have been utilized to examine and qualify flow mechanics of the SCS. In ex vivo porcine eyes, immediately after an SCS injection, the injectate rapidly spreads towards the posterior pole from the scleral spur to the macula, which differs from the variable spread seen in injecting a localized bolus within the vitreous body.17 This posterior and circumferential fashion in which the injectate spreads within the SCS has been confirmed through endoscopic visualization.10 Thus, suprachoroidal delivery may expose the injectate to a much larger surface area of retinal tissue compared to that seen in intravitreal delivery. These advanced examination and imaging techniques highlight the potential advantages of suprachoroidal therapy, including targeted delivery to affected retina-RPE-choroid-sclera (RCS) tissues, compartmentalization for improved safety, and bioavailability.
Suprachoroidal delivery of steroid, anti-VEGF, and gene vector therapies
Suprachoroidal injection continues to be a route of interest for various therapies, including steroid, anti-VEGF and gene therapy. A recent phase II/III trial (Iranian Registry of Clinical Trials identifier: IRCT20200314046761N1) investigated the efficacy of suprachoroidal delivery of triamcinolone acetonide (SCTA) with intravitreal delivery of bevacizumab (IVB) into eyes with centre-involving DMO (CI-DMO).18 Results suggest that the combination of SCTA and IVB injections, rather than just intravitreal monotherapy with IVB, can provide better visual acuity improvement and greater reduction in retinal thickness and macular volume compared to IVB monotherapy in patients with CI-DMO. In addition, the combination therapy, when analysed under a mixed-model, resulted in a significantly higher resolution of macular volume compared to monotherapy. Phase III of the PEACHTREE study (ClinicalTrials.gov identifier: NCT02595398) investigated the efficacy of CLS-TA in patients with macular edema secondary to non-infectious uveitis.19 This trial demonstrated that compared to sham control, treatment with CLS-TA led to significant improvements in BCVA, reduction in CST, resolution of macular edema, and decreased need for rescue therapy compared to the control group. As an extension, a post-hoc analysis showed significant improvement of BCVA and CST in subjects treated with CLS-TA regardless of if they were on concurrent systemic therapy or not.20 CLS-TA (XIPERE®; Clearside Biomedical, Inc., Alpharetta, GA, USA) recently became the first United States Food and Drug Administration (FDA) approved therapy to be delivered through the suprachoroidal route.
Suprachoroidal delivery has also found meaningful utilization in the administration of gene therapy for both inherited retinal diseases as well as DMO and wet AMD. Regenxbio’s phase II AAVIATE trial (ClinicalTrials.gov identifier: NCT04514653) tests the safety and efficacy of suprachoroidal delivery of RGX-314 (an adeno-associated virus 8 [AAV8] vector that codes for an anti-VEGF fab transgene) as treatment for wet AMD.21 Results showed stable visual acuity and central retinal thickness with decreased treatment burden overall. Regenxbio’s ALTITUDE trial (ClinicalTrials.gov identifier: NCT04567550) investigated RGX-314 for treatment in eyes with DR without DMO. Phase II of ALTITUDE results show that after 6 months at ascending dosages, treatment eyes show greater improved vision of ≥2-steps on the ETRDS scale, greater improved Diabetic Retinopathy Severity Scale (DRSS), and fewer cases of DRSS worsening compared to the observed controls.22
Suprachoroidal delivery of small molecule therapies
In the context of drug delivery, a small molecule refers to a low-molecular-weight compound that is typically less than 800–900 daltons in size. These molecules are characterized by their small size and relatively simple chemical structure, allowing them to be easily synthesized, purified and formulated from natural organic resources into relatively affordable therapeutic drugs.23,24
One such medication in the category of small molecules is axitinib, a tyrosine kinase inhibitor that is approved for the treatment of renal cell carcinoma. Its anti-angiogenic effects were evident in preclinical trials in porcine eyes (Figure 3).25 Axitinib acts as a ubiquitous VEGF receptor blocker which may prove to be more effective than traditional anti-VEGF agents that have a narrower and more limited route of effect on angiogenesis. The OASIS trial (ClinicalTrials.gov identifier: NCT04626128) is a phase I/IIa study of the safety and efficacy of the suprachoroidal delivery of a proprietary axitinib injectable suspension (CLS-AX) for visually stable patients with wet AMD who have received at least two previous intravitreal anti-VEGF therapy.26 The OASIS trial consists of 4 cohorts that all underwent a 3-month dose-escalation study, and cohorts 2–4 continued further for another 3 months in an extension study, where they were observed for long-term tolerability, durability, and effect on anatomy. Both study arms showed no adverse events nor events that required treatment. Its 6-month extension study demonstrated that in cohort 3 (n=7) and cohort 4 (n=4), after being treated with one respective dose of CLS-AX, all subjects (11/11) required no additional aflibercept injection for the first 3 months. Six-month data after administration of CLS-AX shows an average reduction of 77%–85% in the number of necessary additional CLS-AX injections when compared the average number of aflibercept injections the patients needed to receive during the 6 months before axitinib administration (Figure 4). Eyes in cohort 3 and 4 shows stable visual acuity and stable central subfield thickness.26
Figure 3:Preclinical data shows a significant reduction in retinal neovascularization after suprachoroidal administration of CLS-AX in porcine eyes25
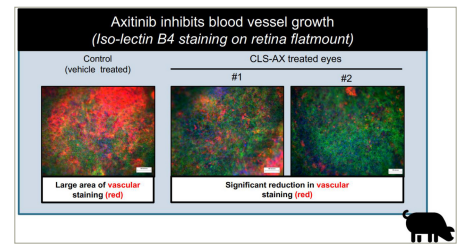
Image reused with permission from Clearside Biomedical, Inc.
CLS-AX = proprietary axitinib.
Figure 4: Anti-VEGF treatment burden reductions seen in eyes treated with suprachoroidal administration of CLS-AX27
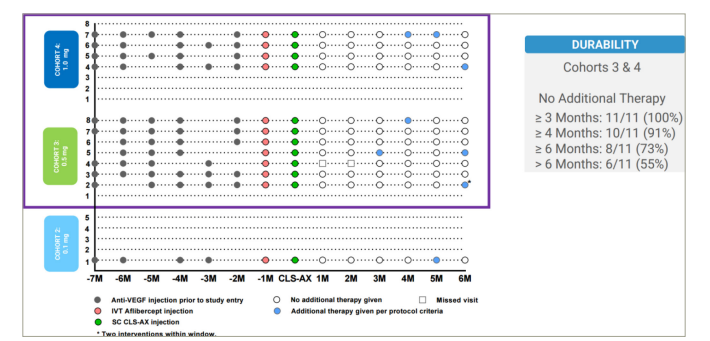
Image reused with permission from Clearside Biomedical, Inc.
IVT = intravitreal; M = month; SC CLS-AX = suprachoroidal injection of proprietary axitinib; VEGF = vascular endothelial growth factor.
A future study on suprachoroidal administration of CLS-AX is intended through the ODYSSEY trial (ClinicalTrials.gov identifier: NCT05131646) where its safety and efficacy are compared to that of intravitreal administration of aflibercept.26 Enrollment has recently started as of June 2023, and the prospective study design will include 60 patients; 40 patients in the CLS-AX arm and 20 patients in the aflibercept arm.
Integrin antagonists, another class of small molecules, selectively inhibit integrin-mediated signalling pathways, thus disrupting neovascularization, reducing inflammation and mitigating retinal vascular leakage.28 Integrin antagonists have also shown to be protective on RPE by downregulating leukocyte-induced cellular death. Rat models have shown that integrin ligands fibronectin and vitronectin have a structural role in vascular formation and the development of choroidal neovascularization.29 Current therapeutic candidates of this mechanism include intravitreal risuteganib (ALG-1001, Allegro Ophthalmics, LLC, San Juan Capistrano, CA, USA), intravitreal THR-149 (Oxurion NV, Leuven, Belgium), and topical SF-0166 (SciFluor Life Sciences Inc., Boston, MA, USA), which have shown promise in treating DME and wet AMD in recent clinical studies.30 CLS-301, a suprachoroidal-delivered small molecule suspension of an integrin antagonist, is being developed. Preclinical studies show suprachoroidal delivery of CLS-301 as having high durability and bioavailability as well as eliciting targeted and compartmentalized distribution.31
Although the plasma Kallikrein-kinin system (PKKS) is the central mechanism behind hereditary angioedema, targeting this pathway has also been shown to play a role in treating DME. The administration of selective PK inhibitor ASP-440, another small molecule, has been seen to improve retinal vascular abnormalities and reduce retinal vascular permeability (RVP) in diabetic rats.32 Additionally, intravitreal injection of C1-INH (C1 esterase inhibitor)decreases RVP in diabetic rats, further suggesting the involvement of local intraocular PKKS activation in elevated RVP in diabetic rats. BCX4161 is a selective inhibitor of plasma kallikrein activity. A preclinical study conducted by Clearside Biomedical involved administering a suprachoroidal injection of BCX4161 suspension bilaterally to rabbits and monitoring drug levels in various ocular structures including RPE, retina, and choroid over 12 weeks.33 The concentrations of BCX4161 in the retina were significantly higher than the in vitro IC99 levels, indicating effective targeting and inhibition of plasma kallikrein. As seen with many drugs delivered suprachoroidally, results showed sustained and high exposure of BCX4161 in the retina-choroid, with promising therapeutic levels and tolerability. Suprachoroidal delivery of BCX4161 demonstrates interesting potential as a safe and effective long-acting small molecule therapy for DME.
Although the eye is immune privileged, immunologic insults play a large role in the progression of proliferative retinal diseases. Specifically, the complement cascade contributes to dry AMD and its advancement to geographic atrophy (GA) without definitive evidence pointing to which of the three complement pathways contributes the most to dry AMD.34 Intravitreal complement system inhibitors such as pegcetacoplan (SYFOVRE™, Apellis Pharmaceuticals, Inc., Waltham, MA, USA) and avacincaptad pegol (IZERVAY™, Iveric Bio, Inc., Cranbury, NJ, USA) recently secured FDA-approval for the treatment of GA secondary to AMD.35 Exploring further into complement inhibition, a preclinical study evaluated the safety and pharmacokinetics of suprachoroidal delivery of A01017, a complement factor D inhibitor, in a rabbit model of dry AMD found that a single bilateral suprachoroidal injection of A01017 was well-tolerated, with minimal toxicity observed.36 The drug exhibited sustained high exposure in the RCS and retina throughout the 92-day study period at all three tested doses. The elimination of A01017 from RCS followed a first-order kinetic with estimated half-lives ranging from 66–76 days. The drug levels in RCS and retina were 3–5 orders of magnitude higher than the in vitro IC90 value, indicating effective inhibition of the alternative pathway of complement activation. Negligible levels of A01017 were detected in the vitreous, aqueous humour, or plasma. These findings suggest that suprachoroidal delivery of A01017 could potentially be a long-acting treatment option for dry AMD, warranting further investigation in its development as a small molecule complement inhibitor.
Conclusion
Suprachoroidal drug delivery presents a unique approach in retinal therapy, offering distinct advantages in drug distribution, safety and convenience. By directly targeting the site of action, suprachoroidal delivery enables sustained and localized drug exposure to retinal tissues, reducing the need for frequent treatment. This approach may eliminate the need for frequent intravitreal injections or systemic medications, which can be burdensome and inconvenient for patients, resulting in a reduced visual benefit in real world conditions. Beyond the well-studied VEGF-related pathways, a multitude of other pathways have been investigated to understand their role in proliferative retinal diseases. These pathways can be effectively targeted through leveraging the mechanisms of small molecules. Small molecules possess the unique ability to easily penetrate tissues and to selectively target molecular pathways involved in diverse ocular diseases. By inhibiting crucial enzymes, receptors, or signaling pathways implicated in disease progression, small molecule therapies hold promise for enhancing therapeutic outcomes. Suprachoroidal delivery of small molecules emerges as a particularly promising strategy, providing targeted and sustained drug administration to the posterior segment of the eye. This localized and prolonged drug exposure holds potential for reducing treatment burden, improving patient compliance, and optimizing therapeutic efficacy across a range of retinal diseases.







