Aetiology
Neurotrophic keratitis arises from damage to the sensory (trigeminal) nerve fibres that innervate the cornea. The cornea is the most densely innervated structure of the body, and the effect of decreased corneal sensitivity can have profound consequences for the health of the corneal surface. As a result of decreased corneal sensation, there is decreased lacrimal gland fluid production, blink frequency and neurotrophic factor synthesis, each of which can contribute to the corneal epithelial breakdown, which is the hallmark of NK.
Impaired corneal innervation can be secondary to systemic disease, most commonly due to diabetes and associated neuropathy. With the increasing prevalence of diabetes and diabetic complications across many countries, there is an increasing burden of ocular surface disease and NK-related complications.1 Other common non-ocular aetiologies of NK include ischaemic or compressive damage to the trigeminal nerve. NK can also be secondary to ophthalmic disease, such as prior herpetic keratitis, chronic dry eye disease or contact lens overuse, among others.2 Although there are many causes of trigeminal nerve damage, the clinical appearance and management of NK does not differ, and therefore we can address the management for all aetiologies of NK collectively.
Staging
The common goal of management of NK, regardless of disease severity, is to preserve long-term vision, reduce permanent corneal damage and, ultimately, to reverse NK-associated changes to the cornea. The Mackie classification defines the clinical spectrum of NK and aids the selection of treatment options to suit the disease severity in an affected eye. Stage I is defined by decreased corneal sensation with punctate epitheliopathy, and treatment, primarily with topical agents, aims to prevent epithelial erosion. Treatment for stage II can include more procedural intervention in addition to the medical therapies used in mild NK; treatment focuses on re-epithelialization of persistent epithelial defects. Progression of corneal erosions to corneal melting and perforation are seen in stage III disease, which requires the most urgent and aggressive interventions in line with its severity in order to prevent loss of the eye.2,3
Treatment of comorbid conditions
An important pillar of NK treatment is to address underlying or comorbid conditions contributing to worsening epitheliopathy. Superimposed infection of the cornea can cause additional inflammation and should be treated with antibiotics. Infection should be ruled out in cases of non-healing epithelial defects because the infection will prevent appropriate wound healing. Oral macrolides such as azithromycin or topical antibiotics can be appropriate, although additional preservatives in topical antibiotic drops can further irritate the ocular surface.
Other common causes of inflammation in patients with NK are dry eye disease and meibomian gland dysfunction. Dry eye can be managed with some of the same topical treatments as NK, such as artificial and autologous tears; however, more specific treatments to reduce dry eye-associated inflammation are readily available. The calcineurin inhibitor cyclosporine is available in two formulations approved for dry eye (Restasis™ 0.05% solution, Allergan, Irvine, CA, USA; Cequa™ 0.09% suspension, Sun Pharmaceutical Industries, Inc., Cranbury, NJ, USA). Lifitegrast (Xiidra™, Novartis AG, Basel, Switzerland) is another, newer medication altering T-cell-mediated immunity and inflammation on the ocular surface. Omega-3 fatty acid and coenzyme Q supplementation have been shown to decrease inflammation associated with meibomian gland dysfunction.4,5
Topical steroids may also provide significant benefit in reducing surface irritation, especially in the long-term management of dry eye disease. However, it is crucial that any possible underlying infection is ruled out prior to steroid initiation in order to avoid worsening the condition; steroids can worsen corneal melt and accelerate the patient towards corneal perforation. Therefore, steroids are to be used with caution.
Current topical treatments for neurotrophic keratitis
Topical treatments for NK can be divided into those that improve lubrication, nutrition and wound healing. Whereas the majority of topical treatments are initiated during milder stages of NK, newer biological agents are available that specifically aim to reduce the stromal tissue loss seen in severe NK cases.
Tears
Artificial tears are a first-line treatment in all stages of NK. There are a variety of preservative-free polymer-based formulations that provide similar functions. Increased lubrication reduces both the shear forces sustained with blinking and the concentration of proinflammatory cytokines on the ocular surface, thus promoting epithelialization.2,6
Serum tears, whether autologous or allogeneic, are closer in composition to real tears compared with artifical lubricants, and have been shown to be effective in NK treatment.7 These eye drops contain nutrients including sugars, proteins and vitamins, which promote epithelial growth and regeneration.8 Platelet-enriched formulations of plasma tears have been shown to stop corneal thinning in neurotrophic ulcers.9 While artificial lubricants are relatively inexpensive and available over-the-counter, serum eye drops are more expensive given the cost of blood draws and special compounding by a local pharmacist. Therefore, serum tears are generally reserved for cases that are refractory to artificial tears. In patients with systemic disease with circulation of autoantibodies, extra consideration should be given to the risk that the ocular manifestations of these diseases may be worsened with use of autologous tears.2
Eye drops derived from cord blood and amniotic membrane are effective in the treatment of NK, and have additional benefits compared with adult serum-derived drops.10–12 Both cord blood and amniotic membrane drops have growth factors that penetrate the cornea, increase cell growth and decrease lymphocytic activation. This immunosuppressive effect is unique to drops derived from umbilical cord and amniotic membrane because they contain immune-modulating peptides and proteins that are absent or found in very low concentrations in adult serum.11 However, due to the source material, these drops are more expensive and difficult to obtain, although a single sample of cord blood can be used for multiple patients.
Biological agents
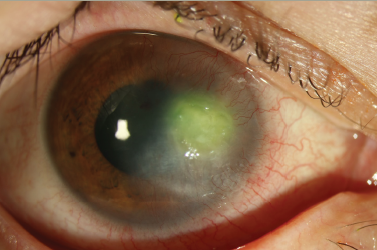
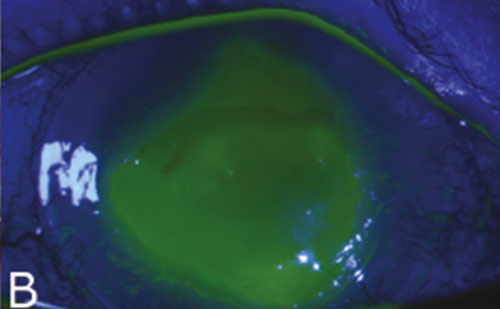
Cenegermin (Oxervate®, Dompé Pharmaceuticals SpA, Milan, Italy) is an eye drop containing recombinant human nerve growth factor (rhNGF) and has been approved by the US Food and Drug Administration for patients with stage II and stage III NK (Figure 1). rhNGF has been shown in the REPARO and NGF0214 trials to maintain corneal epithelial and limbal stem cells and promote reinnervation.13,14 Patients had significant improvement in corneal sensitivity and tear production even up to 2 years following the initial 8-week treatment period.15 The ongoing DEFENDO study is investigating long-term cenegermin results at up to 30 months.16
Matrix-regenerating agent (RGTA) drops (Calcicol®, Laboratoires Théa, Clermont-Ferrand, France; available only in Europe) are a topical treatment that mimics extracellular matrix scaffolding (heparan sulfate) on the ocular surface to promote stromal regeneration. RGTA drops have been trialled in patients with neurotrophic ulcers, and have been shown to be efficacious in decreasing mean ulcer area with 2–3 weeks of treatment.17,18 The French UNICOL study is a randomized controlled trial evaluating RGTA treatment in chronic corneal ulcers; complete results are not yet available.19
A summary of pharmacological interventions for treatment of NK is shown in Table 1.7,12–14,16,19–24
Current procedural treatments
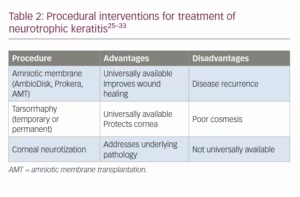
Amniotic membrane
Amniotic membranes are an extremely effective tool for many ocular surface diseases, including NK. Amniotic membrane contains many growth factors that promote corneal epithelial healing and reduce inflammation and scarring.25 There are both in-office and operating room applications. For clinic use, AmbioDisk (Corza Medical, Parsippany, NJ, USA) is a dehydrated sterilized human amniotic membrane allograft. It is as easy to place on the eye as a soft contact lens and does not require sutures. Prokera™ (BioTissue, Miami, FL, USA) is a similar amniotic membrane-based product but is incorporated into a symblepharon ring. These biomaterial products are both effective in improving epithelial disease and healing non-healing epithelial defects in NK. Of note, it has been reported that once the amniotic membrane dissolves, there can be recurrence of disease.26
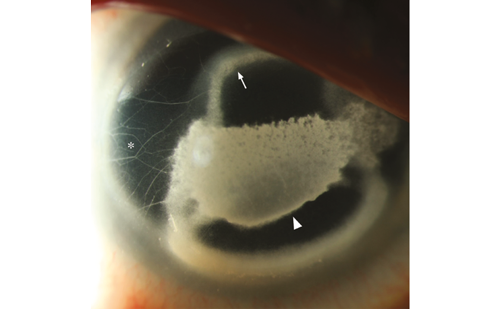
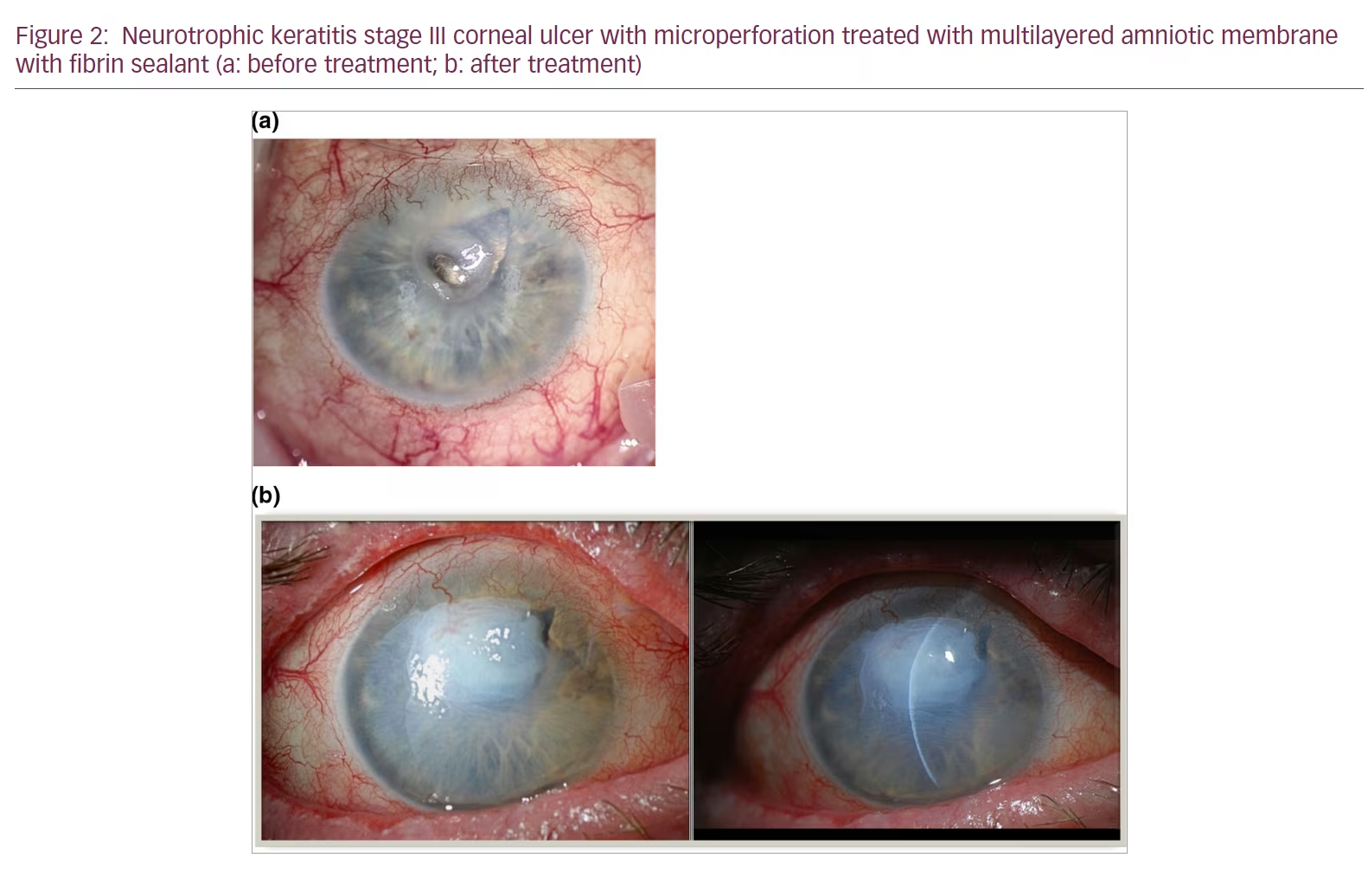
In more severe cases, amniotic membrane transplantation, single or multilayered, can be done in the operating room. Amniotic membrane transplants have been shown in many case studies to be effective in accelerating healing of the epithelium in NK and other ocular surface diseases (Figure 2)27,28
Tarsorrhaphy
Patients with reduced corneal sensation have reduced blink rates, and as a result the ocular surface dries out and can break down. It would then make sense that tarsorrhaphy would provide a solution to this problem. For severe cases of NK with repeated corneal epithelial breakdown, stromal thinning and scarring, temporary or permanent tarsorrhaphy can provide a very effective solution. However, tarsorrhaphy is often not a long-term solution due to cosmetic and social implications.28
Cornea neurotization
Corneal neurotization refers to surgical procedures that aim to restore the lost sensory innervation to the cornea, either via direct nerve transfer or nerve graft from a distant site. Advances in this technique over the past 10 years have made it an increasingly attractive option, offering a potential cure for patients with neurotrophic keratitis.
Direct corneal neurotization was first described by Terzis et al., who found success in restoring corneal sensation by dissecting and transecting the contralateral supraorbital or supratrochlear nerves.29 Incisions were then made on the bulbar conjunctiva to graft the nerves around the corneal limbus. Since then, this technique has been modified to a less invasive endoscopic procedure.30 Indirect transfers of second site autografts have also been described, with the grafts commonly being harvested from the sural nerve.31 Small case series show promising results with improved corneal sensation, as well as improvement in visual acuity and corneal epithelium stability.32,33
Corneal neurotization is a very exciting and promising development in the long-term management of NK, but the techniques are still evolving and it is not universally available as it requires a high level of specialization and a multidisciplinary team approach. Additionally, there is still a paucity of large-scale studies and long-term data on outcomes and longevity of the reported benefits. A summary of procedural interventions for the treatment of NK is shown in Table 2.25–33
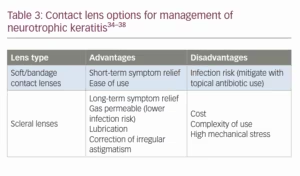
Contact lenses
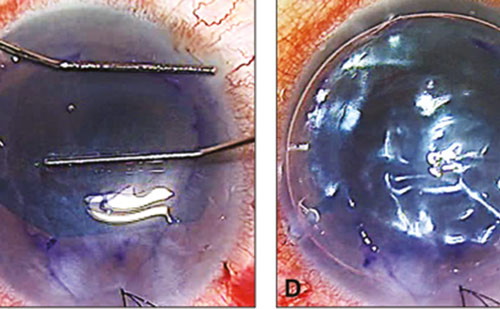
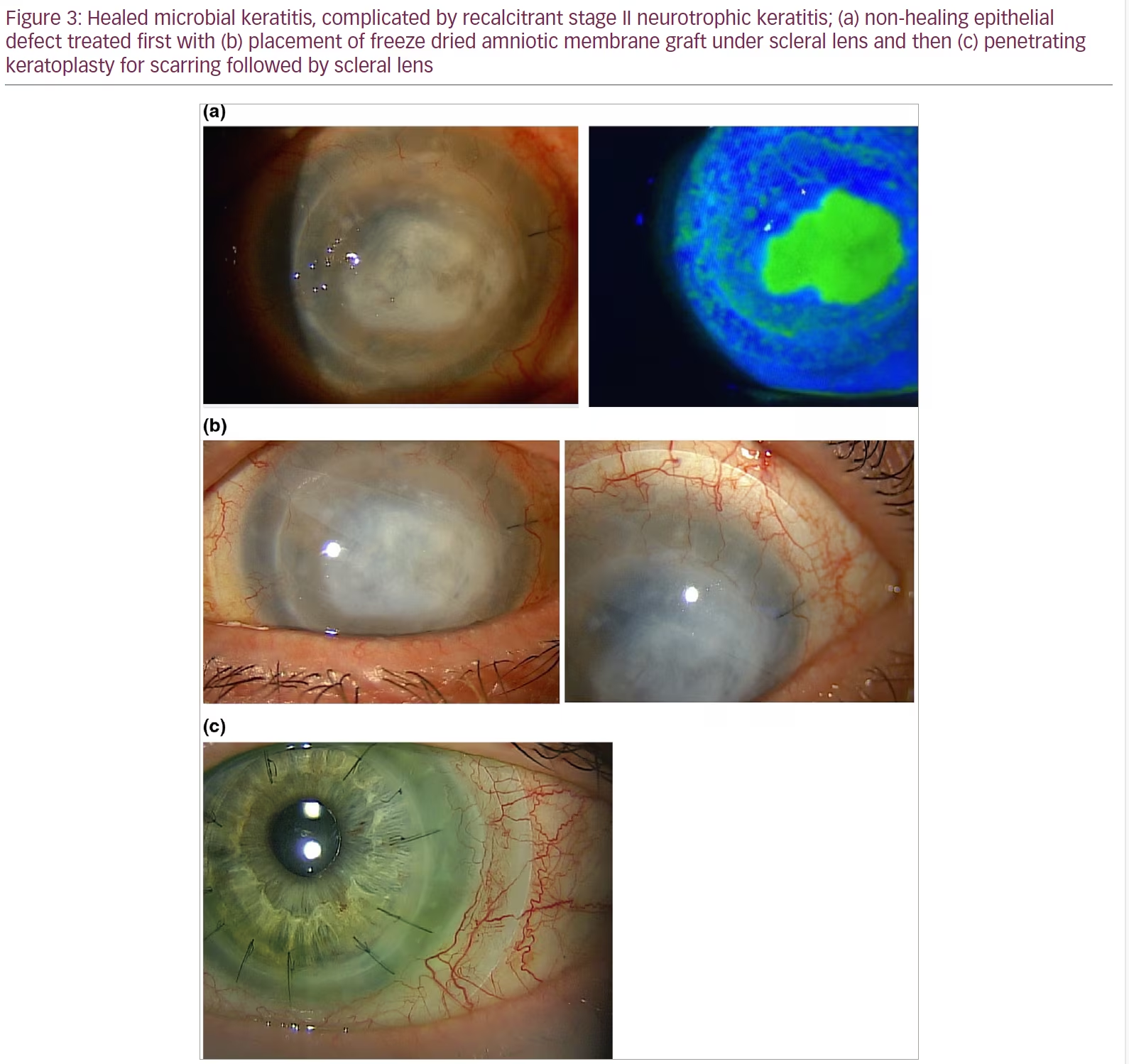
There are a variety of roles that contact lenses can play in the management of NK. Soft contact lenses, or bandage contact lenses, can provide barrier protection to the cornea in the presence of a non-healing epithelial defect, and can accelerate the time to healing.34,35 A better long-term option for a patient with NK would be scleral contact lenses. Scleral lenses are large-diameter rigid gas-permeable lenses that sit on the sclera and vault over the corneal surface, bathing it in a reservoir of fluid. This intervention not only keeps the ocular surface lubricated and protected, but it can also eliminate irregular astigmatism and improve visual acuity in patients with irregular corneas. Scleral lenses have revolutionized the treatment of ocular surface disease. Not only do these lenses promote healing of non-healing epithelial defects, but they can also reduce the risk of further corneal breakdown, thinning and subsequent perforation or scarring (Figure 3).36,37
However, contact lenses come with their risks as well, and patients need to be appropriately counselled as well as monitored clinically. Soft contact lenses are not good long-term options for patients due to the risk of infection. Although topical antibiotic prophylaxis reduces the risk of infection, a small number of patients on proper antibiotic coverage still develop microbial keratitis while using bandage lenses.38 In fact, even scleral contact lenses can pose an infection risk, especially in a neurotrophic patient who may be unaware that they have developed an epithelial defect. An improperly fitted scleral lens can cause eye redness, irritation and even corneal oedema. In a patient with significant corneal thinning, the mechanical stress of applying and removing the lens can even, in rare cases, result in corneal perforation. Finally, high prices as well as complexity of use still pose significant barriers to use for many patients.
In addition to contact lenses, moist chamber goggles can be used as an adjunctive device to limit tear film evaporation and desiccation of the ocular surface, although they have not been studied specifically in the context of NK. There are many commercially available options, and in the inpatient setting makeshift moist chambers can be fashioned from adhesive dressing films. A summary of contact lens options is shown in Table 3.34–38
Treatments on the horizon
Varenicline nasal spray was approved by the FDA for dry eye disease in 2021 (0.03 mg, Tyrvaya®, Oyster Point Pharma, Inc., Princeton, NJ, USA) and is being studied for efficacy in NK. Varenicline acts on acetylcholine receptors of the afferent parasympathetic nerve fibres to stimulate increased lacrimal tear production. In previous studies of intranasal varenicline 0.3 mg and 0.6 mg, the majority of patients had significant increases in tear production measured with Schirmer test score after 8–12 weeks.20,39,40 The Olympia study is an ongoing phase II multicentre trial investigating intranasal varenicline 0.12 mg for symptom relief in stage I NK.41
Topical insulin is a low-cost, widely accessible medication that is being studied for efficacy in NK. In diabetic rats, topical insulin was shown to improve wound healing.42 Until recently, data on the use of topical insulin in human eyes was limited to case reports.43–45 However, Soares et al. described re-epithelialzation of 90% of persistent epithelial defects and chronic ulcers in a small group of patients with insulin treatment.21 An ongoing study at the University of Alberta is comparing the efficacy of insulin drops versus tarsorrhaphy in 60 patients with K.22
Thymosin beta 4 (Tβ4, RGN-259) is a naturally occurring polypeptide that has wide-ranging effects, including promotion of stem-cell recruitment and migration, inhibition of inflammation and apoptosis, and prevention of microbial growth.46 A recent study of 18 patients by Sosne et al. comparing the efficacy of 0.1% Tβ4 eye drops with placebo showed a trend towards efficacy of corneal healing, and significant improvements in patient symptoms.23 The SEER-2 trial is an upcoming phase III trial to investigate the efficacy Tβ4 versus placebo in a larger cohort (70 patients).24
Conclusion
Treatment of NK is largely focused on prevention of progression of disease to improve patient comfort and avoid loss of vision. Although NK has previously been reported as rare, there is a growing body of literature addressing the management of this degenerative keratopathy. In recent years, more therapeutic options have been developed to target the loss of sensation, signalling and growth factors that arise from trigeminal nerve damage.