Keratoconus is the most common corneal ectasia. It disproportionally affects children and adolescents and involves a focal weakening of the biomechanical integrity of the cornea.1 The disease typically manifests as a progressive thinning and bulging of the cornea, which causes the characteristic cone-like protrusions, resulting in increasing myopia, irregular astigmatism and ever-worsening quality of vision.1–3 Historically, the visual symptoms of keratoconus were treated with rigid gas permeable or scleral contact lenses, but these do nothing to stop the progression of the disease.4 Keratoconus progression can eventually lead to corneal hydrops and potentially even rupture, and historically, advanced cases of keratoconus were ultimately treated with keratoplasty.4
The origins of corneal cross-linking
In 1997, Spörl et al. published a new technique that could slow or halt the progression of keratoconus by increasing the biomechanical stiffness of the cornea: corneal cross-linking (CXL).5–7 This technique has become the standard of care for treating keratoconus (and other related corneal ectasias) and has dramatically reduced the need for keratoplasty since its introduction.8–11
The original ‘Dresden protocol’ CXL procedure (named after the city in which it was first developed) involves the removal of the corneal epithelium to enable the stroma (the layer directly beneath the epithelium, which imparts most of the cornea’s structural strength) to be soaked with a photosensitizer – riboflavin (vitamin B2).7 Once saturated with riboflavin, the stroma is irradiated with ultraviolet-A (UV-A) light. Dresden protocol CXL specifies that a total UV-A dose (fluence) of 5.4 J/cm2 is delivered using irradiation at an intensity of 3 mW/cm² over 30 minutes. UV-A energy photoactivates the riboflavin and generates reactive oxygen species (ROS), which oxidatively react and covalently cross-link molecules (predominantly collagen and glycoproteins) in the cornea.12 The cross-linking renders the cornea biomechanically stiffer, counteracting the biomechanical weakening effects of the ectasia; furthermore, through a process called steric hindrance, it renders the cornea more resistant to protease digestion, which may be involved in the pathogenesis of ectasias. As a side benefit, the ROS generated during the UV-A/riboflavin photochemical reaction also damage the cell membranes and nucleic acids of any pathogens present, which has led to the evaluation of CXL for the treatment of corneal infections.13 It is worth noting that riboflavin acts to shield deeper regions of the cornea (and the rest of the eye beneath) from UV-A energy but is consumed during the process, meaning that the stroma is cross-linked from the top downwards.
The Dresden protocol was viewed for many years as the ‘gold standard’ protocol for preventing ectasia progression and is the protocol with the greatest body of evidence supporting its efficacy in treating corneal ectasias.14–16 However, the Dresden protocol has several drawbacks. First, it is extremely time-consuming. Second, epithelium removal and its regrowth can be uncomfortable and painful for the patient, can cause the occurrence of transient haze, and involves a small increase in the risk of postoperative infection during the approximately 1-week period when the epithelium is regrowing. These drawbacks can be managed effectively with pain management protocols and adherence to postoperative topical anti-infective and steroid drug regimens. However, this protocol has another limitation: a minimum stromal thickness limit of 400 µm. This limit was put in place to ensure that a 70 µm region of the posterior stroma was left as an uncross-linked safety margin to protect the corneal endothelium from exposure to damaging levels of UV energy. However, this had the consequence that many eyes with keratoconus that could benefit from the corneal biomechanical strengthening effects of CXL did not receive the procedure.
Historical approaches to cross-linking thin corneas
In 2009, Hafezi et al. used hypo-osmolar riboflavin to preoperatively swell thin corneas to an approximate thickness of 400 µm (Figure 1).17 One drawback of this approach is that the ensuing increase in stromal thickness is highly variable between corneas, making the final corneal thickness after swelling unpredictable.17 Nevertheless, the biomechanical strengthening effect of this approach on the cornea is good, and this technique remains the most frequently used approach to date.18
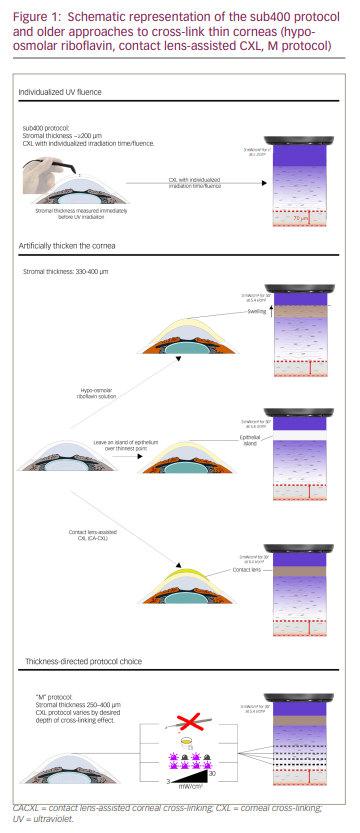
In 2012, Jacob et al. introduced another therapeutic possibility for thin corneas: contact lens-assisted corneal cross-linking (CACXL; Figure 1).19 Again, the idea was to thicken corneas artificially – originally between 350 and 400 µm thick – using ultraviolet barrier-free soft contact lenses soaked in iso-osmolar riboflavin.19 However, our group has shown that the biomechanical strengthening effect of CACXL is lower than that of Dresden protocol cross-linking.20 The reason for this is that, depending on the contact lens used, the physical presence of the lens on the cornea reduces available atmospheric oxygen by almost 50% compared with the cornea being exposed to the air.20 As a result, the procedure decreases the long-term modulus (corneal stiffening effect) by 15% to 20%.20 Wollensak et al. also showed that the biomechanical effect of CACXL in porcine eyes is approximately one-third less than after standard CXL.21
A third approach has been to leave islands of epithelium over the thinnest areas of the corneal stroma (Figure 1).22 However, the interface between the epithelial island and abrased regions of the cornea has the potential to refract UV-A energy into the intermediate stroma and produce unpredictable cross-linking effects. This appears to be the case, as epithelial island CXL results in an unequal demarcation line between epithelialized and de-epithelialized areas.
What is worth noting is that all of these approaches involve adapting the cornea to the technique rather than the technique to the cornea.
Characterizing the corneal cross-linking reaction
When the Dresden protocol was first developed over 20 years ago, the UV-riboflavin cross-linking reaction was not fully understood. For example, the 3 mW/cm² UV intensity specified in the protocol resulted from the technical limitations of mercury vapour UV lamps, which were the only suitable UV light sources available at the time. The advent of LED-based UV-A light sources meant that higher-intensity light sources became available. This raised the possibility that irradiation times could be shortened if UV-A intensity was increased, as predicted by the Bunsen-Roscoe law of photochemistry, which states that the overall photochemical effect of a reaction should remain the same when the same total energy (fluence) is used.23 However, these accelerated protocols failed to achieve the same level of stiffening that the lowerpintensity, slower Dresden protocol achieved: the more the reaction was accelerated, the lower the resulting stiffening effect.24,25 The reason for this was published by our research group in 2013: oxygen is consumed rapidly by the UV-A-riboflavin photochemical reaction, and the pace of the reaction is limited by the ability of oxygen to diffuse into the cornea.20,26,27 This also explained the suboptimal stiffening effect of CACXL in thin corneas: the contact lens acted as a barrier to oxygen diffusion into the cornea.
Nonetheless, the realization that oxygen was an essential component of the cross-linking reaction meant that the UV-A-riboflavin photochemical reaction and the depth of its effect in the stroma during cross-linking could be accurately modelled. This finding allowed our group to generate an algorithm that could predict the depth of cross-linking based on the measured depth of a patient’s cornea with atmospheric oxygen, Dresden protocol-style epithelial removal and UV irradiation at 3 mW/cm².28 This algorithm, called ‘sub400’ (Figure 1), incorporates Fick’s law of diffusion, estimates of riboflavin and oxygen diffusion, and UV energy exposure calculations using the Lambert-Beer law of light absorption.29,30 The sub400 algorithm could therefore be used to calculate individualized irradiation durations that would generate the desired depth of the cross-linking effect. This raised the possibility that thin corneas could be cross-linked, retaining a 70 µm uncross-linked safety margin at the base of the stroma, without resorting to methods that modify corneal thickness. In other words, CXL could be adapted and individualized to the cornea rather than adapting the cornea to the technique.
The sub400 protocol in clinical practice
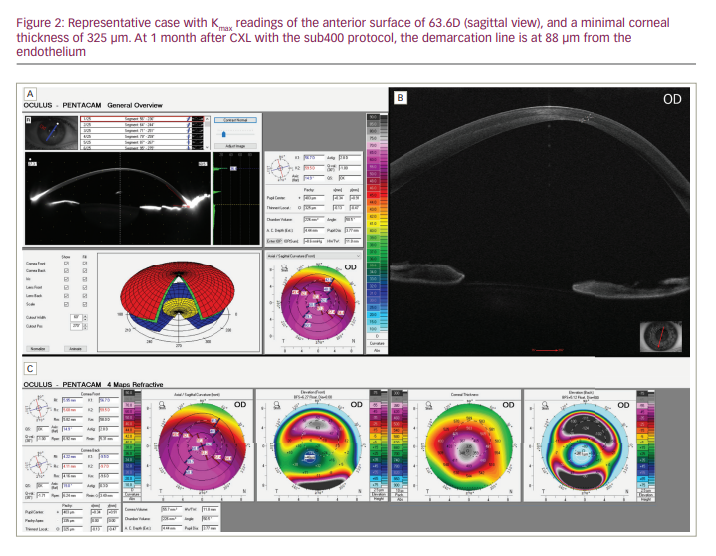
Recently, a case series was published that described the results of 39 patients with progressive keratoconus and stromal thicknesses of ≤400 µm (average: 343 µm; range: 214–398 µm) who were treated with the sub400 protocol.31 In the study, each patient’s corneal pachymetry was measured with a hand-held ultrasound pachymeter after epithelial cell debridement; then, a customized UV irradiation time, which was selected using a look-up table, was used at an intensity of 3 mW/cm².31 The primary endpoint was the prevention of keratoconus progression 1 year after the procedure. It is worth noting that many of these corneas would have been considered too thin to cross-link by the previous thin cornea cross-linking protocols; furthermore, these patients would almost certainly have required corneal transplantation, yet the eyes of 90% (35/39) of the patients showed tomographical stability after 12 months. No corneas showed signs of decompensation, which is in keeping with recent experimental evidence suggesting that the current threshold of endothelial damage might have been overestimated for decades.32 The algorithm also appeared to work well, as a significant correlation (p=0.004) was observed between irradiation time and demarcation line depth. A representative case of a thin cornea treated with sub400 protocol cross-linking is described in Figure 2.
The M protocol approach to cross-linking thin corneas
It is worth noting that, as a rule, modifications to the Dresden protocol cross-linking approach almost universally result in a shallower cross-linking effect.33 Historically, protocols that accelerate UV irradiation resulted in a lower depth of cross-linking, as demonstrated by the demarcation line, as oxygen availability is the rate-limiting step in the UV-riboflavin photochemical reaction; moreover, accelerating these protocols with higher irradiation intensities consumes oxygen faster than it can diffuse into the stroma. Furthermore, transepithelial, or epi-on, CXL has also resulted in shallower cross-linking effects. Irrespective of whether iontophoresis or penetration enhancers like ethylenediaminetetraacetic acid, trometamol or benzalkonium chloride are used to get riboflavin to pass through the epithelium, the intact epithelium still constrains oxygen diffusion and absorbs UV energy, reducing the depth of cross-linking effect; furthermore, it is worth noting that each approach results in a different cross-linking depth.34
These observations led Mazzotta et al. to propose a protocol that integrates all available evidence on the demarcation line depths achieved by different cross-linking protocols and to suggest the appropriate protocol for any given corneal depth: the ‘M’ protocol (Figure 1).34 However, this protocol has the drawback that surgeons would require UV light sources that can deliver energy at multiple intensities, as prescribed by these protocols (from 3–30 mW/cm²), using both continuous and pulsed light protocols; furthermore, it would require them to have other equipment (e.g. iontophoresis apparatus) available to be able to perform all the procedures listed. By contrast, the sub400 protocol requires only a single cross-linking device that can deliver the standard Dresden protocol 3 mW/cm² intensity, thus making it a far simpler proposition.
The future of corneal cross-linking
As mentioned above, there are several drawbacks associated with the Dresden protocol that many research groups have been working on overcoming. In our view, these alternatives will almost certainly be applied to the treatment of thin corneas. The ectasia-stabilizing effects of CXL protocols that leave the epithelium in place are also now approaching that of Dresden protocol cross-linking, as are high-fluence, high-intensity accelerated protocols.35 We have recently shown in the laboratory that high-intensity accelerated CXL that delivers higher fluences than the Dresden protocol’s 5.4 J/cm² can provide Dresden protocol-like corneal strengthening.36 As preclinical and clinical experience with these protocols increases, the sub400 protocol can be further extended and validated to incorporate these advances. It is already the case that there is a high-fluence sub400 protocol update that involves 9 mW/cm² UV-A irradiation intensities (Hafezi et al., manuscript in preparation). The sub400 protocol has also been used successfully to cross-link a keratoglobus cornea using a slit lamp-based cross-linking method.37 Cross-linking at the slit lamp is an interesting approach because it permits CXL to be straightforwardly performed in an office or procedure room setting at the near-ubiquitous slit lamp, which brings cost, resource and general access to the procedure benefits relative to CXL performed in an operating theatre.38
Conclusions
The customization of CXL protocols to individual patient stromal thicknesses and the corresponding adaption of irradiation time using the sub400 protocol has simplified the process of cross-linking thin corneas. In addition, the sub400 protocol has expanded the procedure to patients with ultra-thin corneas – as low as 200 µm – that were too thin to be cross-linked by previous CXL protocols. Future advances in CXL protocols can also be adapted to the sub400 protocol, meaning that thin corneas can continue to be cross-linked safely and accurately in a customized manner using future gold-standard procedures, whatever they may be.