Acanthamoeba is an opportunistic, unicellular, free-living protist with at least 25 species and 23 genotypes so far identified (on 18S rRNA sequencing).1 Organisms have a two-stage life cycle: metabolically active trophozoites and quiescent double-walled cysts. Both trophozoites and cysts are small, usually 15–50 µm in length for trophozoites and 5–30 µm for cysts.2 Exposure to stress results in rapid encystment of Acanthamoeba, which are resistant to harsh environmental conditions and can lay dormant for years. Trophozoites are typically well treated with most antimicrobial therapies; however, treatment of the cysts requires specific cysticidal medications over a long period of time.
Acanthamoeba causes corneal infections through a multistep process beginning with epithelial breakdown, often through microtrauma from contact lens wear. This microtrauma and increased expression of epithelial glycoproteins allows Acanthamoeba to adhere and release various enzymes and toxins that degrade the stroma.3 In particular, the binding is mediated by mannose-binding protein, along with two laminin-binding proteins, to the epithelial glycoproteins.4 Acanthamoeba then releases ecto-adenosine triphosphatases involved in caspase-3 activation, neuraminidases,5 phospholipase, elastase, glycosidase and metalloproteases, which work in concert to have a cytotoxic effect.4 Although Acanthamoeba trophozoites can penetrate the Descemet membrane, intraocular infection rarely occurs because of the intense neutrophil response in the anterior chamber.6 Trophozoites have been shown to demonstrate a chemotactic response to cells of neural crest origin, leading to infiltration of the corneal nerves and painful keratoneuritis.7
Epidemiology and risk factors
Acanthamoeba is found ubiquitously in water, air and soil, including swimming pools, hot tubs and ponds. It is also found frequently on contact lenses and contact lens cases, especially when not correctly sanitized.8 Common human exposures to these environments, especially in lower socioeconomic settings, trauma and recent increases in contact lens use have contributed to an increasing number of cases of Acanthamoeba keratitis (AK).1 The infection rate is estimated to range from 1–2 per million adults or 2–10 per thousand contact lens wearers.9 These estimated rates are highly variable due to the different prevalence of contact lens use, the amoebicidal efficacy of contact lens cleaning systems and the use of diagnostic tests for AK.
Clinical features
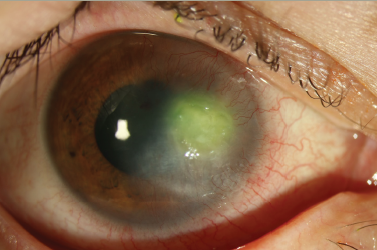
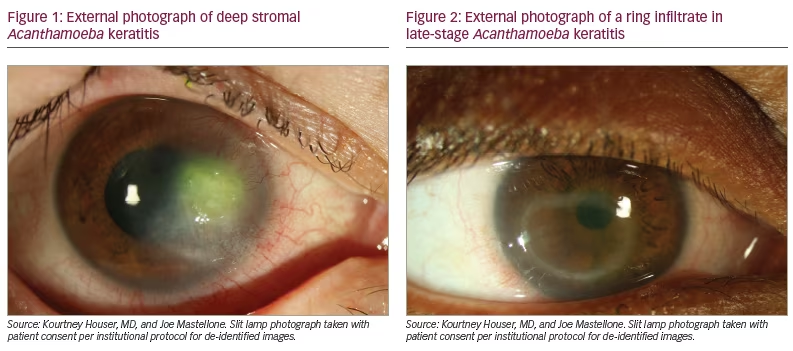
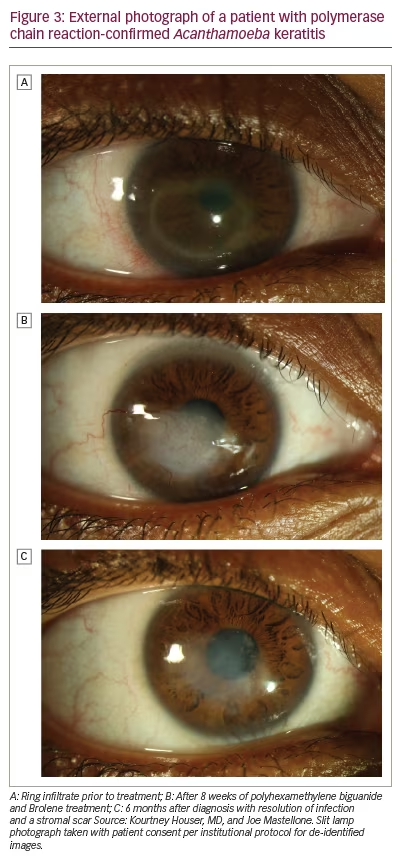
AK should be considered in any presentation of keratitis, especially those associated with contact lens use or exposure to soil or water. Patients typically report subacute eye pain, photophobia and blurred vision. The pain is often out of proportion to examination findings due to the presence of keratoneuritis. Tu et al. described five levels of AK severity: epitheliitis (with grey/dirty epithelium), epitheliitis with radial neuritis, anterior stromal disease, deep stromal keratitis (Figure 1), or ring infiltrate (Figure 2), which is typically only seen in late stages of the disease.10 Rarely, Acanthamoeba can also cause scleritis, chorioretinitis, endophthalmitis, adnexal disease or disseminated encephalitis.11 As a result of the wide array of clinical presentations, the early epitheliitis stages can easily be confused with herpes simplex keratitis, whereas in the advanced stage, the deeper infiltrates can resemble bacterial or fungal keratitis.12 As late diagnosis is associated with worse outcomes, a high index of suspicion, recognition of both typical and atypical findings, lack of improvement with alternative treatments (especially herpetic), and ancillary testing are recommended to provide timely and accurate diagnosis.2
Diagnosis
The gold standard of diagnosis for AK is culture of corneal scrapings. This is typically done in a media with inactivated Escherichia coli, and examination for Acanthamoeba trails is then performed under light microscopy. Acanthamoeba will move away from the inoculation point to ‘graze’ on the E. coli.13 Co-infection with Acanthamoeba is common, possibly due to the presence of other bacteria, fungi, algae and viruses that Acanthamoeba feed on or have an endosymbiotic relationship with.13 Various stains such as acridine orange and calcofluor white can assist with the diagnosis on culture or smears. However, cultures are only positive in 52–67% of cases, possibly due to inadequate sample material, previous antimicrobial treatments and deeper stromal infection, and even lower rates have been reported; furthermore, calcofluor white works only for cysts.14 Even positive culture results are often delayed due to the slow-growing nature of Acanthamoeba. Contact lenses may be cultured when there is a high index of suspicion, but they should not be routinely cultured, as Acanthamoeba colonization has been found in an estimated 7–8% of contact lens storage cases used by asymptomatic individuals.15
Confocal microscopy is another valuable diagnostic tool that allows microscopic examination of individual corneal layers at a 2–4 µm resolution. Confocal microscopy is also noninvasive and rapid, and can be standardized for longitudinal examinations in the same patient to assess for progression. Studies have shown variable sensitivity (~60–90%) for confocal microscopy, which is likely to be due to the operator-dependent yield.16 The specificity is typically higher (~80–93%) due to the characteristic findings such as signet ring-shaped bodies, bright cyst with a dark background, hyperreflective bodies with spindle-like projections, and others.2 However, caution must be taken due to the similar appearance between cysts and inflammatory cells.17 Additionally, as confocal microscopy requires the patient to be imaged directly, it is not available at most institutions.
Polymerase chain reaction of scrapings or biopsies can also identify the presence of amoebic DNA in a tissue sample after amplification of amoeba RNA in the laboratory.3 This process shows excellent specificity (99% or greater), similar to that for cultures, but also a higher sensitivity (73–87%); however, it is significantly more technical and expensive.18
Treatment
Medical treatment
Medical treatment of AK requires treatment of both the active trophozoite form and the stress-resistant cyst form.3 The trophozoite form is sensitive to a variety of antibiotics, antifungals, antiprotozoals and even antineoplastic therapies. However, the cystic form classically requires prolonged antiseptics such as polyhexamethylene biguanide (PHMB) and chlorhexidine (biguanides) or propamidine (also known as Brolene; Thornton & Ross, Linthwaite, UK) and hexamidine (diamidines).3 These positively charged molecules bind to the phospholipid layer of the Acanthamoeba, disrupting the cell membrane and denaturing cytoplasmic proteins.19 Patients should be counselled that treatment often lasts several months and possibly up to a year (Figure 3).
PHMB and chlorhexidine have been shown to have relatively low minimal cysticidal concentrations, allowing treatment of AK at low doses with relatively minimal damage to the corneal epithelium.20 However, prolonged use can still lead to epithelial toxicity. PHMB is typically dosed at 0.02% to 0.06%, chlorhexidine at 0.02% to 0.2%, propamidine at 0.1% and hexamidine at 0.1%, concentrations that are nearly 100-fold lower than when used as antiseptics.20
Bacterial co-infection is reported to be as high as 40%, and it is therefore common for initial treatment to include antibacterial drops as well as anti-AK drops.21 Many antibacterial treatments also have activity against the trophozoite form of Acanthamoeba.
For refractory cases, success has been reported with the addition of voriconazole. Topical 1% voriconazole has been shown to have good outcomes when added as an adjunctive treatment,22 and studies have also shown success with topical or oral voriconazole monotherapy.23,24 Patients starting voriconazole should be counselled on the possibility of transient visual phenomena or hepatic toxicity.
Miltefosine is an alkylphosphocholine originally used for leishmaniasis that also selectively disrupts Acanthamoeba cell membranes. A few case series have shown success for refractory AK; however, there appears to be a high rate of initially worsened inflammation.25 Topical or oral steroids are often needed to treat the inflammatory response. Patients should also be counselled on gastrointestinal side effects such as nausea, vomiting, abdominal pain and diarrhoea, as well as the possibility of liver and renal toxicity.
The use of topical steroids is controversial in AK. Although it is commonly accepted that the use of topical steroids before the diagnosis and treatment of AK is associated with worse outcomes (due to increased trophozoite expression),26,27 there may be a role for delayed topical steroids as an adjunct therapy to control inflammation. McClellan demonstrated in vivo that steroids increase the excystment of Acanthamoeba into the active trophozoite form, which allows the infection to be more sensitive to treatment but also increases the pathogenicity.28 Various case series have shown similar or noninferior outcomes in patients who received delayed topical corticosteroids, and both topical steroids or systemic immunosuppression therapy may especially play a role in patients who present with scleritis or a hypopyon.27,29,30 If topical steroids are used, it is recommended that patients receive anti-AK therapy for a minimum of 2 weeks before steroid treatment and continue for at least 4 weeks after cessation of steroids.
Procedural and surgical treatments
Although first-line treatment of AK remains medical therapy, procedural or surgical treatment can be added to facilitate treatment or as a last resort for refractory cases. For example, thorough epithelial debridement for early AK can help reduce pathogen load and also assist with penetration of topical therapy.3
In a case series by Kandori et al., four cases of superficial stromal AK that were resistant to medical therapy for a week were successfully treated with laser phototherapeutic keratectomy, with excellent visual outcomes.31 Patients underwent ablation to an average depth of 254 µm until infectious tissue was no longer seen.31
A few case series in refractory cases have reported success with riboflavin cross-linking with ultraviolet A light32 or rose bengal photodynamic antimicrobial therapy (RB-PDAT) with green light.33 Both processes are theorized to generate free radicals that have significant antimicrobial properties without significant corneal toxicity.34 In a study by Naranjo et al., 10 patients with AK unresponsive to standard medical therapy for at least 2 weeks were treated with 1–3 rounds of RB-PDAT, with success in seven patients.33 In a meta-analysis by Papaioannou et al., 11 patients were treated with ultraviolet A cross-linking, with success in 10, although five required repeat treatment.35 Although reports of these therapies are promising, there are currently insufficient data to recommend their incorporation into standard practice.
Therapeutic penetrating keratoplasty (PK) has been performed to help debulk infectious load but outcomes were poor and complicated by infection recurrence, graft rejection or graft failure from glaucoma.36 In a series of 32 therapeutic PK cases, Kashiwabuchi et al. reported a 40% rate of glaucoma, 56% rate of graft failure and 38% rate of a second PK.37 As medical therapy is generally successful, therapeutic PK is now typically reserved for severe refractory cases or for severe thinning or perforation. Anti-AK therapy is typically continued pre- and postoperatively, adopting a regimen that minimizes epithelial toxicity on the new graft. PK or deep anterior lamellar keratoplasty have also shown good outcomes in treating scarring and irregular astigmatism after quiescent AK.38
Prognosis
Prognosis is highly dependent on the severity of disease at presentation and treatment initiation. In a review of 349 patients, 90% of patients had a final vision of 20/40 or better, although ~5% lost all useful vision.20
Conclusions
AK is a potentially devastating infection of the ocular surface that is increasing in frequency due to contact lens use and ubiquitous environmental contamination. The clinical features of AK and imperfect confirmatory testing present a unique diagnostic challenge where progression has a significant impact on prognosis. We recommend a high index of suspicion for Acanthamoeba in contact lens wearers, especially those who have been diagnosed with herpetic keratitis. We also recommend recognition of typical and atypical symptoms, along with early and multimodal testing with culture and polymerase chain reaction or confocal microscopy if possible. Medical therapy with biguanides and diamidines are often effective, although involve subjecting the patient to a long and painful treatment course. Exciting new strategies to manage refractory AK have been reported with voriconazole, miltefosine or topical steroids, which have been shown to be valuable adjuncts. Procedural interventions such as phototherapeutic keratectomy, cross-linking or PDAT have also shown promise, but the small-sample case series are insufficient to make recommendations for clinical practice. Ultimately, earlier awareness and evaluation for AK in patients with corneal infections can lead to better outcomes and a more tolerable clinical course.