Combined cataract-vitreoretinal surgery (also known as phacovitrectomy) consists of pars plana vitrectomy (PPV), phacoemulsification and implantation of an intraocular lens (IOL). Phacovitrectomy can be performed by a single vitreoretinal surgeon or by an anterior segment surgeon working in tandem with a vitreoretinal surgeon. Combined surgery can be advantageous in certain patients as it overcomes some limitations of sequential surgery. Alternatively, the procedures can be performed sequentially in two different stages, with vitrectomy followed by cataract surgery or vice versa.
The decision of whether to perform combined surgery depends on the practice setting, surgeon preference, and the significance of the cataract and vitreoretinal pathology. This article discusses pertinent preoperative considerations and challenges of cataract surgery in previously vitrectomized eyes, and highlights outcomes for phacovitrectomy in phakic patients with epiretinal membranes (ERMs), macular holes, retinal detachments (RDs), and vitreous haemorrhage (VH) due to proliferative diabetic retinopathy (PDR).
Vitrectomy and cataract formation
Cataract formation is the most common visually significant complication of vitrectomy. It is hypothesized that nuclear sclerotic cataract progression after vitrectomy may be related to light toxicity, oxidation of lens proteins, gas exposure and damage from free radicals, while the formation of posterior subcapsular cataracts may be associated with the use of intraocular tamponade.1–5 Phacovitrectomy eliminates the need for a second surgery, may improve the visualization for fine retinal surgical manoeuvres, and allows retinal and visual recovery to occur unimpeded by secondary cataract development.
Considerations for staged surgery
Surgical considerations
Cataract surgery in vitrectomized eyes is more challenging and associated with a higher risk of intra- and postoperative complications. Vitrectomized eyes may have occult zonular compromise and posterior capsule damage, leading to excessive mobility of the lens–iris diaphragm. The lack of vitreous support also causes larger fluctuations in the anterior chamber depth, which makes capsulorrhexis and phacoemulsification more technically difficult.6–10
A retrospective case–control study by Biró and Kovacs, involving 84 previously vitrectomized eyes undergoing cataract extraction, found that posterior capsular fibrosis was the most common intraoperative complication, occurring in 24% of eyes.8 Posterior capsule rupture occurred in 9.5% of eyes.8 In a prospective study by Pardo-Muñoz et al., involving 100 eyes that underwent cataract surgery after PPV, the authors reported posterior capsule tears in 4%, zonular dehiscence in 5%, luxated nucleus into the vitreous chamber in 2% and retinal re-detachment in 4% of eyes.7 In 2009, Cole and Charteris performed a retrospective analysis of 72 patients undergoing cataract extraction who had previously received vitrectomy with intraocular tamponade for RD.9 The authors reported that 12.5% of patients experienced intraoperative events, including capsulorrhexis tears, zonular dehiscence and vitreous loss. Retinal re-detachment occurred in a concerning 5.6% of eyes.9 There is also evidence in the literature to suggest that vitreoretinal pathology, such as macular hole, RD and VH, may worsen or recur after cataract surgery in previously vitrectomized eyes.11–13
Time to recovery and logistical burden
The need for subsequent cataract surgery after vitrectomy prolongs the recovery course and delays the final visual outcome, which may negatively affect patient satisfaction. Furthermore, the significant time and cost associated with staging two separate surgeries increases the logistical and financial burden on patients.
Refractive outcomes
While the overall visual rehabilitation process may take longer in patients undergoing sequential vitreoretinal-anterior segment surgery, the refractive outcomes tend to be more predictable. The stability of the retinal thickness and morphology following vitrectomy allows for higher quality calculations to be obtained prior to cataract surgery, which may allow the surgeon to consider refractive correction with a toric or even a multifocal lens.
Considerations for combined phacovitrectomy
Surgical considerations
In patients with significant cataract, combined phacovitrectomy, in which the anterior segment surgeon addresses the cataract first, optimizes the view and surgical access to the retina. However, some intraoperative challenges must be considered when coordinating combined surgery. The vitreoretinal and anterior segment surgeons must be cognizant of the positioning of incisions and port sites in order to avoid hindering one another. Complications with one part of surgery may render it unsafe to proceed with subsequent steps. Corneal oedema after phacoemulsification can make visualization during vitrectomy difficult, and conversely, complications such as VH or posterior capsule damage during vitrectomy may create obstacles for cataract extraction. Moreover, combined surgery tends to be associated with a higher risk of postoperative intraocular inflammation, posterior synechiae and posterior capsule opacification.6–10
Time to recovery and logistical burden
Combined phacovitrectomy leads to an overall faster recovery time as it prevents subsequent cataract development, which interrupts and prolongs visual rehabilitation. Port et al. performed a single-institution, retrospective study, which demonstrated that phakic patients with ERM undergoing phacovitrectomy attained visual recovery 15 months sooner than patients who underwent sequential surgery.14 Furthermore, they found that the total time spent in the operating room and associated costs were significantly lower in the combined surgery group.14 However, not all eyes that undergo vitrectomy develop a visually significant cataract. Thus, for this subset of patients, combined surgery may be riskier and less cost effective. In certain cases, combined surgery can be beneficial to patients as it limits the number of times they have to undergo anaesthesia. This is especially important in patients who have cardiopulmonary or other systemic diseases that necessitate general anaesthesia. Having to undergo only one surgery also incurs less financial burden for patients, though depending on the institution, combined surgery may not be reimbursed as well as sequential surgery for each provider. Furthermore, it may be difficult for two surgeons to set aside time to perform a combined vitreoretinal-anterior segment procedure.
Refractive outcomes
In any cataract surgery with concurrent vitrectomy, the refractive outcome will not be as predictable, as the effective lens position will change due to removal of the vitreous. The importance and feasibility of attaining a good final refractive outcome must be considered when determining whether a patient should undergo combined surgery. Some studies have demonstrated that phakic eyes undergoing phacovitrectomy are at higher risk of having a postoperative myopic result. Liu et al. compared phakic eyes with macular hole undergoing phacovitrectomy versus cataract surgery alone and found that eyes in the combined surgery group were more likely to have a myopic result.15 Kim et al. evaluated phakic eyes with RD that underwent either phacovitrectomy or sequential surgery and also concluded that eyes in the phacovitrectomy group were more likely to have a postoperative myopic surprise.16
In addition to having less predictable refractive outcomes, combined phacovitrectomy may also limit the available options for IOLs. As a precise measure of the effective lens position and axial length is difficult to obtain in phakic patients with concurrent vitreoretinal pathology, multifocal and extended-depth-of-focus lenses are typically avoided. Toric lenses, however, may be utilized in cases when the surgeon can confirm the final IOL position at the end of the vitrectomy. Toussaint et al. reported a consecutive series of 55 eyes undergoing simultaneous cataract surgery with toric IOL implantation and PPV, and demonstrated that the final IOL axis was within five degrees from target in 85% of eyes and within 15 degrees from target in 95% of eyes.17 The authors concluded that the most important factor in obtaining a good refractive result was being vigilant in verifying the final IOL axis at the end of the procedure.17
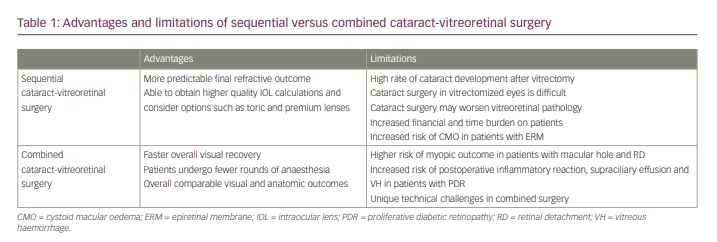
Table 1 summarizes the advantages and limitations of sequential versus combined cataract-vitreoretinal surgery.
Specific pathologies to consider
Epiretinal membrane
In eyes with a visually significant cataract and ERM of unclear visual significance, many surgeons prefer to stage the procedure, starting with the cataract and proceeding with the ERM only if vision remains limited postoperatively. It is important to consider the possible effects cataract surgery may have on ERM development, although it is unclear whether cataract surgery definitively accelerates ERM progression as there are conflicting data in the literature. A study by Hayashi and Hayashi found no significant difference in foveal thickness in patients with ERM who underwent cataract surgery compared with those who did not (7.0% versus 5.3%, respectively).18 However, other studies suggest that cataract extraction in patients with ERM may worsen traction, macular thickness and metamorphopsia. Multiple studies have demonstrated increased rates of cystoid macular oedema (CMO) following cataract surgery in eyes with preexisting ERM compared with those without ERM.11,19–21 In the study by Schaub et al., 15.7% of eyes with preexisting ERM developed postoperative CMO compared with only 5.9% of eyes without ERM.11 Similarly, the retrospective study by Hardin et al., involving 812 eyes with ERM and 159,184 reference eyes, found that eyes with ERM were at significantly higher risk of postoperative CMO compared with eyes without ERM (8.6% versus 1.38%, respectively).20
Due to concern for ERM progression and CMO development after cataract surgery, consideration should be given to combined phacovitrectomy in eyes with ERM, particularly when the ERM appears visually significant. Studies evaluating the safety and efficacy of combined surgery for ERM have been promising. Dugas et al. performed a multicentre, retrospective, comparative case study involving 174 phakic eyes with ERM undergoing either combined or sequential surgery, and concluded that both approaches led to equivalent functional and anatomic outcomes, with the combined surgery group having faster visual recovery.21 Yiu et al. performed a similar study involving 81 eyes that underwent either combined surgery or ERM peel only.22 Although the authors reported equivalent functional and anatomic outcomes between the two groups, there was a non-statistically significant trend for ERM recurrence (p=0.084) and need for repeat surgery (p=0.096) in the eyes that underwent phacovitrectomy.22 A prospective trial by Hamoudi et al. allocated 62 phakic eyes with ERM to three different groups: group 1, cataract surgery and subsequent PPV; group 2, PPV and subsequent cataract surgery; or group 3, phacovitrectomy.23 There were no significant differences in the final best corrected visual acuity (BCVA), refractive error and central macular thickness among the three groups. Although group 1 was found to have a higher incidence of CMO, 17% of patients who underwent cataract surgery alone did not need to undergo subsequent PPV. This finding suggests that there may be a benefit to deferring vitrectomy until the outcome of the cataract surgery is known. The authors also compared corneal changes among the three groups and found no significant difference in the percentage decrease in endothelial cell count at 12 months postoperatively (15.3%, 20.0% and 19.3% for groups 1, 2 and 3, respectively).24 They concluded that performing cataract surgery before, after or in tandem with PPV did not have a significant effect on endothelial cell density, central corneal thickness or corneal volume.24
Macular hole
Macular hole formation is a rare complication of cataract surgery and is thought to be due to anteroposterior tractional forces inducing a detachment of the posterior cortical vitreous. Additionally, there are concerns that previously repaired macular holes may reopen during phacoemulsification. This consideration is important when determining whether to stage or combine phacoemulsification and vitrectomy in phakic eyes with macular hole. In 2007, Hager et al. performed a retrospective analysis of 65 eyes with previously repaired macular holes that underwent cataract surgery.25 They reported no cases of hole reopening following cataract surgery and concluded that cataract surgery did not worsen the reopening rate of macular holes.25 In contrast, a study by Bhatnagar et al. found that cataract extraction in eyes with a history of repaired macular hole may increase the risk of the hole reopening.12 In this case series, 211 eyes with idiopathic macular holes were divided into four groups: group 1, prior cataract extraction; group 2, vitrectomy followed by cataract extraction; group 3, vitrectomy only; and group 4, vitrectomy and cataract extraction. The authors found that the greatest rate of macular hole reopening occurred in eyes that underwent cataract surgery after previous successful vitrectomy for macular hole (20% of eyes). Furthermore, they noted that CMO development after cataract surgery was associated with a sevenfold increased risk of the macular hole reopening.12
Overall, results from trials evaluating the safety and efficacy of combined surgery for phakic patients with macular holes have been favourable. Lahey et al. performed a case series involving 89 phakic eyes with macular hole that underwent phacovitrectomy and found that 89% of holes were successfully closed.26 Of the nine failed cases, four patients had successful hole closure with one additional surgery. In 2014, Savastano et al. performed a retrospective, interventional case series involving 565 eyes with ERM, vitreomacular traction or macular hole undergoing either PPV alone or combined phacovitrectomy.27 The authors concluded that there was no increase in postoperative complications in the combined surgery group. In 2021, Savastano et al. also published a prospective, comparative study involving 110 eyes with ERM or macular hole undergoing either PPV alone or combined phacovitrectomy.28 The authors concluded there were no significant differences in visual outcomes and postoperative complications between the two group.
Some studies suggest that phacovitrectomy may expedite visual recovery. Muselier et al. performed a retrospective series involving 120 phakic eyes with macular hole undergoing either combined surgery (64 eyes) or sequential surgery (56 eyes).29 The authors found that combined surgery led to faster visual recovery, with only the phacovitrectomy group achieving a significant improvement in BCVA at 6 months. Both groups had a significant improvement in BCVA at 12 months. Overall, 100% and 96% of holes were closed in the combined and sequential groups, respectively.29
Refractive outcomes are also important to consider in these cases. Liu et al. performed a retrospective study evaluating the refractive outcomes in phakic eyes with macular holes that underwent phacovitrectomy and in phakic eyes without macular holes undergoing cataract surgery alone.15 Patients undergoing phacovitrectomy had a significantly higher risk of attaining a myopic result (67.9% versus 27.3%, p=0.004).15 Repair of the macular hole prior to cataract surgery may allow for improved preoperative measurements of axial length and therefore superior refractive outcomes.
Retinal detachment
In most cases, the urgency of RD repair may lead surgeons to opt for a staged approach rather than combined phacovitrectomy. However, phacovitrectomy must be considered in cases with significant cataract that limits the view for vitrectomy. RD is a known risk after phacoemulsification, and there may also be an increased risk of retinal re-detachment after phacoemulsification in eyes that have undergone prior RD repair.13,30 In a population-based, retrospective cohort study involving 537 eyes with a history of RD repair with scleral buckle, Forsell and Monestam found the 10-year cumulative risk of RD following cataract surgery to be 1%.31 The risk of recurrent RD following cataract surgery in previously vitrectomized eyes has been estimated to be as high as 5.6%.7,9
Several studies have shown favourable anatomic and safety results following phacovitrectomy in phakic patients with RD. A study involving 1,017 eyes with rhegmatogenous RDs undergoing either phacovitrectomy or vitrectomy alone found that additional phacoemulsification during vitrectomy was not associated with higher rates of re-detachment.32 Kim et al. performed a retrospective study involving 56 phakic patients with macula-sparing RD undergoing phacovitrectomy, which showed a 100% final surgical success rate for reattachment.33 In 35.7% of patients, however, the postoperative visual acuity was worse than the preoperative visual acuity owing to ERM development (10.7%), posterior capsular opacification (21.4%) and CMO (3.6%).33 In 2021, Tan et al. demonstrated that eyes undergoing phacovitrectomy and PPV alone had an anatomic success rate of 84.3% and 89.2%, respectively.34
Despite good anatomic outcomes in cases of combined phacovitrectomy, visual outcomes may be limited by postoperative myopic surprise due to difficulty obtaining accurate, high-quality lens calculations. To investigate this issue, Kim et al. performed a retrospective study evaluating the refractive outcomes of combined phacovitrectomy versus delayed cataract surgery after PPV in patients with rhegmatogenous RDs, and concluded that there was a statistically significant risk of induced myopic shift in eyes that underwent phacovitrectomy.16 The authors attributed this to underestimation of the axial length and suggested either delaying cataract surgery or using the axial length in the fellow eye to circumvent a myopic outcome.16 In the study by Tan et al., the authors also found that patients in the combined surgery group had a significantly higher risk of having a myopic outcome (p=0.047), especially in cases of macula-off RD.34
Vitreous haemorrhage in proliferative diabetic retinopathy
Combined phacovitrectomy is frequently necessary in diabetic patients with dense cataracts and VH or tractional RDs in order to allow for visualization and adequate vitrectomy. A combined approach is important in these cases as several studies have demonstrated a risk of retinopathy progression, iris neovascularization, neovascular glaucoma and VH in eyes undergoing cataract surgery alone.35,36 In eyes with mild cataract and diabetic pathology requiring vitrectomy, however, a staged approach can be considered, with cataract surgery following vitrectomy.
Several studies have demonstrated that phacovitrectomy for PDR is safe and efficacious. However, some have suggested that combined surgery may increase the risk of postoperative complications such as supraciliary effusions, fibrinous reaction and VH. Lahey et al. reported an interventional case series involving 233 eyes with PDR (153 with VH, 58 with tractional RD, 12 with macular traction) undergoing phacovitrectomy, and showed good visual outcomes (average increase of 4.3 Snellen lines).37 Overall, 10% of patients required repeat vitrectomy for VH (12 patients) and RD (10 patients).37 Another case series of 138 phakic eyes with PDR (78 with VH, 36 eyes with tractional RD) undergoing phacovitrectomy demonstrated that combined surgery was safe and effective, with only 4.4% of patients needing repeat vitrectomy (3 for VH, 3 for RD).38 Rivas-Aguiño et al. retrospectively evaluated visual outcomes and complications in 48 phakic eyes with PDR undergoing phacovitrectomy versus two-step surgery.39 VH occurred significantly more frequently in the phacovitrectomy group compared with the two-step surgery group (35.7% versus 15%, respectively). The authors concluded that sequential surgery may be advantageous in minimizing postoperative VH, leading to better BCVA outcomes.39 Park et al. performed a retrospective study involving 60 eyes with PDR that underwent phacovitrectomy (30 eyes) versus PPV alone (30 eyes) to evaluate morphological changes in the anterior segment and its associations with postoperative inflammatory complications.40 The risk of supraciliary effusions was significantly higher in the combined surgery group compared with the PPV only group (80% versus 46%). A decrease in angle opening and anterior chamber depth was observed in the phacovitrectomy group, and the incidence of abnormal intraocular pressure and intraocular fibrinous inflammation was significantly higher in the phacovitrectomy group (60% and 30% versus 30% and 7%, respectively). The authors concluded that combined surgery induced more anterior segment morphological changes that were associated with inflammatory complications.40
Billing for phacovitrectomy
A realistic consideration for staged versus combined surgery is reimbursement. For combined phacovitrectomy, the cataract portion is only paid at 50% of the normal current procedural terminology code fee or relative value unit. This includes combined cases where the second surgeon bills under the same tax identification numbers. However, this should be weighed with the logistics of a staged procedure and cost for the patient and surgery centre.
Author recommendations regarding combined phacovitrectomy
Different providers at various institutions have a unique set of risks and benefits to consider when determining whether a patient should undergo combined surgery, as every practice is set up differently. The authors of this review provide patient care at two tertiary academic centres, where scheduling and subspecialty coordination for combined phacovitrectomy is easily achieved. The decision for combined surgery is based on the significance of the cataract as well as the predicted visual impact of the retinal findings. In a setting where the cataract limits the view for treatment of retinal pathology, particularly when treatment of the retinal pathology is time sensitive as in RD, we recommend phacovitrectomy. In the setting where there is a visually significant cataract and retinal pathology with unknown visual significance (such as an ERM), we generally suggest performing cataract surgery first in order to adjudicate whether vitrectomy is indicated to ameliorate the retinal pathology.
In the setting where both the cataract and retinal pathology are significant (especially if the cataract limits the view to the retina), we recommend a combined approach, if possible, based on availability of the surgeons and the operating room. We find that our patients usually prefer this approach for both logistical and financial reasons. However, staging is reasonable if phacovitrectomy is not possible or if the patient has high refractive expectations or the desire for an advanced technology lens, including presbyopia or astigmatism-correcting technologies. In our practices, we generally counsel patients on the limitations of lens options available and discuss realistic expectations regarding their overall visual prognosis based on the severity of their preexisting and current vitreoretinal pathology.
Conclusion
While phacovitrectomy involves careful coordination and may present unique intraoperative challenges, combined vitreoretinal-anterior segment surgery may be very beneficial for certain patients, especially those with transportation issues or those too ill to undergo multiple rounds of anaesthesia. Advancements in vitrectomy and phacoemulsification have allowed combined surgery to be safe and effective while overcoming several limitations of sequential, two-step surgery. As ophthalmic surgeons from different subspecialties continue to collaborate, and surgical techniques and instrumentation for combined surgery continue to improve, providers will be more able to deliver care with the aim of improving efficiency, patient safety and visual outcomes.