Age-related macular degeneration (AMD) is a leading cause of vision impairment and legal blindness in developed countries, with a projected estimate of 288 million cases in 2040.1 AMD can be classified as exudative or non-exudative. The early AMD-related lesion is represented by drusen, which are subretinal lipofuscin accumulations caused by an impaired clearance function of the retinal pigment epithelium (RPE) cells. Drusen are used to classify AMD as the early form (i.e. the presence of several small [<63 μm] or intermediate-sized [≥63 but <125 μm] drusen) or the intermediate form (i.e. the presence of extensive small or intermediate-sized drusen, or any large drusen [≥125 μm]).2,3 Other common early findings are reticular pseudodrusen, also called subretinal drusenoid deposits, which are defined as retinal debris localized above the RPE.2,3 Other possible findings include RPE anomalies (e.g. hypertrophy, hyperplasia, intraretinal migration), pigment epithelium detachment (classified as drusenoid, vascularized or mixed), hyperreflective foci (interpreted either as activated microglia or migrated RPE cells) and acquired vitelliform lesions.4
The onset of macular neovascularization (MNV) (Figure 1) or geographic atrophy (GA) (Figure 2) marks the transition to advanced AMD stages.3 In these cases, further imaging findings include exudation, outer retinal atrophy, inner retinal thinning, fibrosis, choroidal anomalies (e.g. choroidal thinning, cavitations or caverns) and outer retinal tubulations, defined as the round organization of photoreceptor remnants.4
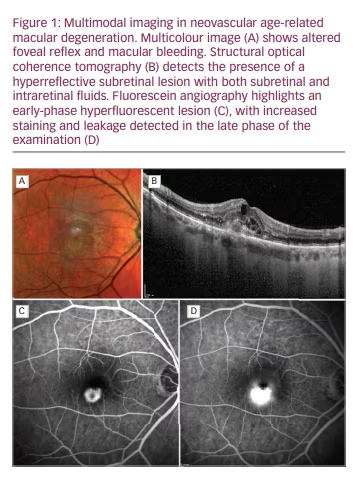
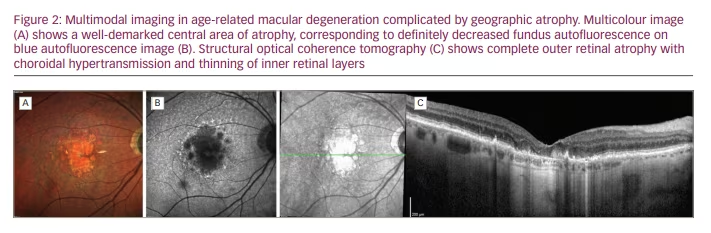
Multimodal imaging is a fundamental tool with which to non-invasively assess microstructural retinal anomalies characterizing all the stages of AMD, and has improved the understanding of AMD pathogenesis and its diagnostic workup.
Pathophysiology and molecular pathways involved in AMD
The pathogenesis of AMD is extremely complex, and includes inflammation, oxidative stress and loss of retinal homeostasis. The Beaver Dam Eye and the Framingham Eye studies highlighted the following risk factors: age, smoking, genetic susceptibility, cardiovascular disease, arterial hypertension, female sex, white race and systemic dysmetabolism.2–5 A major pathogenic cause in AMD onset and progression is uncontrolled release and activity of vascular endothelial growth factor (VEGF). This family of dimeric glycoproteins of approximately 40 kDa includes several isoforms, namely VEGF-A, -B, -C, -D, -E, -F and placental growth factor.6 VEGF production is upregulated by the isoforms of hypoxia-inducible factor-1 and by insulin-like growth factor-1, which play important roles in retinal angiogenesis.7,8 VEGF production is also upregulated by increased hypoxia and oxidative stress.9,10
The main sources of VEGF are RPE cells, astrocytes, Müller cells, endothelium and ganglion cells.11–14 In this context, RPE senescence plays an important role in determining the onset of the first AMD-related signs and in promoting the release of VEGF and of many proinflammatory cytokines.15 Other relevant pathogenic mediators include erythropoietin, angiopoietin (Ang)-1 and -2, and platelet-derived growth factor.16–18 These factors are fundamental in governing AMD progression towards the advanced exudative form.
Patients with GA experience loss of retinal tissue. According to the Classification of Atrophy Meeting nomenclature, retinal atrophy includes the following forms: complete RPE and outer retinal atrophy; incomplete RPE and outer retinal atrophy; complete outer retinal atrophy; and incomplete outer retinal atrophy.19 The pathogenesis of GA is extremely complex, and includes increased oxidative stress, inflammation and upregulation of the complement system.20–24 In addition, GA may occur secondarily to exudative AMD, and an initially pure GA form may be complicated by the onset of MNV.
Current therapeutic approaches to wet AMD
MNV is a common complication that occurs in the advanced stages of AMD. The Consensus on Neovascular AMD Nomenclature Study Group distinguishes the following types of MNV: type 1 (below the RPE, representing the most frequent MNV), type 2 (above the RPE), mixed type 1–2, type 3 (corresponding to the old retinal angiomatous proliferation form) and aneurysmal type 1 (corresponding to the old polypoidal choroidal vasculopathy form).25
A typical phenomenon caused by MNV onset is the presence of exudation. This might occur as intraretinal fluid (detected in ≥67% of wet AMD cases at baseline), subretinal fluid (detected in ≥80% of wet AMD cases at baseline), pigment epithelium detachment and subretinal hyperreflective material.25 However, it should be taken into account that at least 5% of MNV cases are characterized by no signs of exudation for at least 6 months.25
The prognosis of wet AMD radically changed after the introduction of anti-VEGF intravitreal therapies. Although the MNV pathogenesis includes several mediators, as described above, VEGF plays a critical role in the onset and progression of the neovascular network and of exudation. Current anti-VEGF molecules include bevacizumab (Avastin®; Hoffmann-La Roche, Basel, Switzerland), ranibizumab (Lucentis®; Novartis Pharmaceuticals, Basel, Switzerland), aflibercept (Eylea®; BAYER Pharma, Leverkusen, Germany) and brolucizumab (Beovu®; Novartis Pharmaceuticals Canada Inc., Dorval, Canada). Bevacizumab is a fully humanized immunoglobulin (Ig)G1 molecule of 148 kDa that binds VEGF-A isoforms, and is used off-label to manage exudative retinal diseases.26 Ranibizumab is a recombinant humanized immunoglobulin G1κ isotype monoclonal antibody fragment of 48 kDa that also binds VEGF-A isoforms and was the first on-label anti-VEGF treatment for retinal diseases.27 Aflibercept, a type of agent known as VEGF-Trap, is a dimeric glycoprotein of 115 kDa that shows high affinity for VEGF-A isoforms, VEGF-B and placental growth factor.28 Brolucizumab is a single-chain antibody fragment of 26 kDa; it is the newest anti-VEGF molecule with very high affinity for VEGF-A isoforms.29,30 Although it is very efficient in inducing fluid reabsorption, there are some doubts regarding the safety profile of brolucizumab, especially with respect to the risk of ocular inflammatory events; for this reason, brolucizumab is now considered a second-line treatment that requires further study to assess its safety profile.
All of these drugs are effective and safe (with provisos for brolucizumab, as above) for the management of wet AMD, with non-inferiority of different anti-VEGF molecules in managing neovascular complication and exudation.31 The main difference among the anti-VEGF molecules is in their treatment durability; brolucizumab is the longest-acting treatment, with a duration of action of up to 3 months. The current recommendation from the expert panel of the European Society of Retina Specialists is to consider anti-VEGF agents as the treatment of choice for neovascular AMD.32
A major factor in the efficacy of anti-VEGF injections is the choice of treatment regimen. While monthly fixed and as-needed regimens previously represented the schemes of choice, growing evidence supports the superiority of treat-and-extend regimens as a means of improving the long-term efficacy of anti-VEGF agents and visual outcomes for patients.33–35 However, many factors and prognostic biomarkers influence the visual outcomes achieved with anti-VEGF injections, including MNV type and fluid category.36,37 In particular, whereas intraretinal fluid, pigment epithelium detachment and subretinal hyperreflective material are negative prognostic biomarkers, the presence of subretinal fluid alone or its persistence with a thickness of less than 200 µm is associated with a better visual prognosis.38,39
Current therapeutic approaches to dry AMD
In contrast to wet AMD, there are currently no approved treatments for dry AMD (either the early/intermediate or GA forms). The current therapeutic approach mainly involves nutraceutical supplementation, based on evidence from the Age-Related Eye Disease Studies (AREDS) 1 and 2. The formulation studied in AREDS1 contained vitamin C (500 mg), vitamin E (273 mg/473 IU), beta-carotene (15 mg), zinc (80 mg) and copper (2 mg).40 The AREDS2 formulation removed beta-carotene and added lutein/zeaxanthin and omega-3 long-chain polyunsaturated fatty acids.41 In both studies, nutraceutical supplementation was associated with slowing of AMD progression and a reduced probability of atrophic or neovascular complications.40,41
Both subthreshold laser and photobiomodulation approaches have been tested to slow the progression of non-exudative AMD. However, the efficacy of these treatments is still uncertain and they require further validation.42,43
New frontiers in managing wet and dry AMD
The 5-year CATT and SEVEN-UP studies highlighted how the long-term visual outcomes of patients with neovascular AMD treated with anti-VEGF therapies are characterized by an irreversible loss of visual function, caused by the onset of fibro-atrophic complications.44,45 Data such as these stimulated interest in new therapeutic approaches, with the aim of finding more effective therapies with a longer duration of action than currently available molecules. A key point for the long-term management of exudative retinal diseases is the possibility of reducing the burden of treatment, improving the ability of public health systems to manage patients and optimizing the economic and human resources. As such, the main goal of current research is to find treatments with a longer duration of action than current therapeutic approaches, together with superior or non-inferior outcomes.
While research into neovascular AMD is concentrating on making improvements over current therapies, effective treatments for dry AMD and GA are still needed to delay or avoid atrophy progression and irreversible dramatic visual impairment. A complete list of new treatments is reported in Table 1.
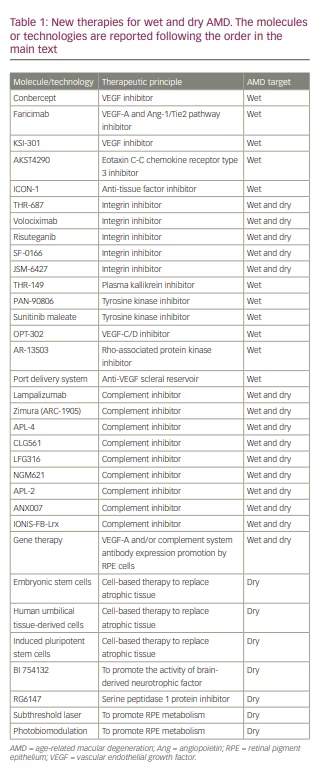
Conbercept (Chengdu Kanghong Biotech Company, Sichuan, China) is a molecule belonging to the VEGF-Trap class. It is obtained from the fusion of the extracellular domain 2 of VEGF receptor 1 and the extracellular domains 3 and 4 of VEGF receptor 2 with the Fc portion of human IgG1. At present, conbercept is only commercialized in China; it was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency for use in clinical trial settings in 2016 and 2017, respectively. Its efficacy in treating neovascular AMD has been investigated in the PHOENIX phase III study,46 as well as in the PANDA-1 (ClinicalTrials.gov NCT03577899) and PANDA-2 (ClinicalTrials.gov NCT03630952) phase III clinical trials.
Faricimab (Hoffmann-La Roche) belongs to the family of designed ankyrin repeat proteins (DARPins), a class of molecules that mimic antibodies and show high affinity for VEGF.47 Specifically, this 150 kDa molecule is characterized by two different targets: faricimab can simultaneously and independently bind and neutralize both VEGF-A and the Ang-1/Tie2 pathway, the latter through an interaction with Ang-2.48 The 36-week AVENUE phase II randomized clinical trial and the 52-week STAIRWAY phase II randomized clinical trial demonstrated the efficacy of faricimab in managing neovascular AMD. Faricimab was non-inferior to monthly ranibizumab in maintaining visual function, with a prolonged treatment interval up of 4 months.49,50 The BOULEVARD phase II and the more recent YOSEMITE and RHINE phase III studies established the efficacy and prolonged durability of faricimab in diabetic macular oedema, confirming its promising role in the management of exudative retinal diseases.51,52 Recently, following the positive results coming from the TENAYA and LUCERNE phase III clinical trials,53 faricimab (Vabysmo™; Genentech, South San Francisco, CA, USA) received FDA approval to treat wet AMD.
KSI-301 (KODIAK Sciences, Palo Alto, CA, USA), is a new-generation antibody biopolymer conjugate resulting from fusion of an anti-VEGF humanized monoclonal antibody with a phosphorylcholine-based polymer. This combination was developed to increase the pharmacological duration of anti-VEGF activity. This molecule is currently under investigation in a phase Ib trial (ClinicalTrials.gov NCT03790852) involving patients with neovascular AMD, diabetic macular oedema and retinal vein occlusion, and in the DAZZLE phase II trial (ClinicalTrials.gov NCT04049266) involving patients with neovascular AMD eyes only. In addition, the phase III BEACON study (ClinicalTrials.gov NCT04592419) is comparing the efficacy of KSI-301 with aflibercept for the management of macular oedema secondary to retinal vein occlusion.
AKST4290 (Alkahest, San Carlos, CA, USA) is an inhibitor of the natural receptor for eotaxin C-C chemokine receptor type 3 (CCR3), a highly expressed molecule in MNV. This molecule is currently under investigation as an oral formulation administered in combination with intravitreal anti-VEGF injections in the AKST4290-201 (ClinicalTrials.gov NCT03558061) and -202 (ClinicalTrials.gov NCT03558074) phase IIa clinical trials.
Carotuximab (DE-122) (SANTEN, Osaka, Japan; TRACON Pharmaceuticals, San Diego, CA, USA), an anti-endoglin antibody (a mediator stimulated by hypoxia and promoting neovascularization), was investigated in a phase IIa randomized controlled trial (ClinicalTrials.gov NCT03211234). In 2020, development was discontinued because the trial did not reach its primary endpoint.
ICON-1 (Iconic Therapeutics, San Francisco, CA, USA) is an anti-tissue factor immunoconjugate protein produced by linking recombinant modified factor VIIIa protein with the Fc portion of human IgG1. Its role is to interfere with the release of anti-tissue factor by MNV. It has been tested in wet AMD, combined with ranibizumab (EMERGE phase II clinical trial; ClinicalTrials.gov NCT02358889)54 and aflibercept (DECO phase II clinical trial; ClinicalTrials.gov NCT03452527).
Integrin inhibitors are a class of molecule developed to contrast the overexpression of integrins in retinal exudative diseases, promoting proliferation and neovascular phenomena. Many integrin inhibitors are currently under investigation, including THR-687 (Oxurion, Leuven, Belgium), volociximab (Ophthotech Corporation, New York, NY, USA; now Iveric Bio), risuteganib (Alg-1001) (Luminate, Allegro Ophthalmics, San Juan Capistrano, CA, USA), SF-0166 (SciFluor Life Sciences, Boston, MA, USA) and JSM-6427 (Takeda Pharmaceutical Company, Tokyo, Japan).55 THR-687 was recently the subject of publications demonstrating its potential therapeutic role in diabetic macular oedema.56 However, it is reasonable to assume a potential role of integrin inhibitors in other exudative retinal diseases as well, including wet AMD. Risuteganib is under investigation in dry AMD (ClinicalTrials.gov NCT03626636).
Plasma kallikrein inhibitors represent a new class of intravitreal drugs developed to interfere with the kallikrein–kinin system, a VEGF-independent pathway promoting inflammation, vasodilation and vascular permeability. A recently completed phase I study of THR-149 (Oxurion) showed that the agent has a good safety and tolerability profile, and was effective in inducing exudation regression in diabetic macular oedema.57 This class of drugs might have a promising role in the management of exudative retinal diseases.
Tyrosine kinase inhibitors are another class of potentially relevant molecules for the management of exudative retinal diseases, acting as inhibitors of the hypoxia-induced VEGF-A and platelet-derived growth factor molecular pathways. PAN-90806 (PanOptica, Mount Arlington, NJ, USA) is a topical tyrosine kinase inhibitor formulation that is under investigation in a phase I/II clinical trial (ClinicalTrials.gov NCT03479372). Sunitinib maleate is an intravitreal tyrosine kinase inhibitor formulation that is under investigation in the ADAGIO phase I/IIa (ClinicalTrials.gov NCT03249740) and ALTISSIMO phase IIb (ClinicalTrials.gov NCT03953079) clinical trials in patients with neovascular AMD.
OPT-302 is a VEGF-C/D inhibitor that is being investigated in combination with ranibizumab injections in a phase IIb clinical trial performed in neovascular AMD patients (ClinicalTrials.gov NCT03345082).
MHU650 (Novartis Pharmaceuticals Canada Inc.) is a new molecule currently under investigation in a phase I study (ClinicalTrials.gov NCT04635800), which is aiming to assess the agent’s safety, tolerability and pharmacokinetic profile in exudative retinal diseases, including neovascular AMD.
AR-13503 is a Rho-associated protein kinase inhibitor that is currently being tested in a phase I study (ClinicalTrials.gov NCT03835884) in patients with neovascular AMD or diabetic macular oedema.
The port delivery system (PDS) (Genentech) is a refillable drug delivery system that is permanently surgically implanted in the sclera and the pars plana. The PDS can be refilled with ranibizumab to guarantee a constant and prolonged administration of the drug, thus reducing the burden of anti-VEGF treatments for patients with neovascular AMD.58 It has been investigated in the LADDER phase II study (ClinicalTrials.gov NCT02510794) and ARCHWAY phase III clinical trial (ClinicalTrials.gov NCT03677934).
Complement inhibitors represent a class of molecules currently under investigation in both the wet AMD and dry AMD settings. As described above, the complement system has a major role in the pathogenesis and progression of AMD.59 With respect to GA AMD, lampalizumab (ClinicalTrials.gov NCT01229215; development discontinued), Zimura (ARC-1905) (phase II/III GATHER1 study60 and phase III GATHER2 study; ClinicalTrials.gov NCT04435366), APL-4 (POT-4/AL-78898A) (ClinicalTrials.gov NCT03525613 and NCT03525600), CLG561 (ClinicalTrials.gov NCT02515942), LFG316 (ClinicalTrials.gov NCT01527500), NGM621 (phase II CATALINA study; ClinicalTrials.gov NCT04465955), APL-2 (DERBY and OAKS phase III studies; ClinicalTrials.gov NCT03525600 and NCT03525613), ANX007 (ClinicalTrials.gov NCT04656561), GEM103 (ClinicalTrials.gov NCT04643886) and IONIS-FB-Lrx (phase II GOLDEN study; ClinicalTrials.gov NCT03815825) are all under investigation in clinical trial settings, with each agent developed to inhibit specific components on the complement system.
Gene therapy is also considered a therapeutic option both for wet and dry AMD. RGX-314 (REGENXBIO, Rockville, MD, USA), an AAV8 vector-mediated approach to induce the production of an anti-VEGF Fab, is under investigation in a phase I/IIa trial (ClinicalTrials.gov NCT03066258). BD311 (BDgene Co., Ltd. Shangai, China) (ClinicalTrials.gov NCT05099094) is VEGF-A-targeting gene therapy that promotes VEGF-A antibody expression by RPE cells to neutralize the activity of VEGF. This agent is currently being investigated in a phase I clinical trial. Many other attempts at gene therapies have been tried and might provide intriguing new perspectives on managing AMD, but the current data indicate that this approach is far from being applied in the real-life clinical setting.61 Recently, gene therapy has also been proposed for dry AMD. FocuS is a phase I/II multicentre trial that is testing the efficacy of GT005, a recombinant, non-replicating, adeno-associated viral vector encoding a human complement factor that has been developed to induce RPE cells to produce a complement system inhibitor (ClinicalTrials.gov NCT03846193).
For GA, cell-based therapies have the potential to replace the atrophic tissue and thus improve visual function. Approaches include using human embryonic stem cells,62 human umbilical tissue-derived cells and induced pluripotent stem cells (ClinicalTrials.gov NCT02286089 and NCT04339764).
Other potential pharmacological approaches for GA are assessing the role of other metabolic targets. BI 754132 (Boehringer Ingelheim, Ingelheim, Germany) is a humanized monoclonal IgG1 antibody that promotes the paths physiologically activated by the brain-derived neurotrophic factor, thus mimicking the TrkB tyrosine kinase receptor (ClinicalTrials.gov NCT04002310). RG6147 (Genentech) belongs to the class of serine peptidase 1 protein inhibitors, and interferes with the production of heat shock serine protease expressed in GA (ClinicalTrials.gov NCT03972709).
Subthreshold laser represents a non-pharmacological therapeutic approach that may interfere with AMD progression and the onset of complications. The principle is based on administering low-power energy – below the threshold of thermal retinal damage – to stimulate RPE cells.63 Subthreshold laser therapy has largely been tested in exudative retinal diseases, including diabetic macular oedema and central serous chorioretinopathy; however, some studies have highlighted a potential role for this laser approach in treating intermediate AMD stages. Indeed, the improvement of RPE metabolism through subthreshold laser stimulus has been associated with the removal of retinal debris, including drusen and reticular pseudodrusen, without progression towards outer retinal atrophy.64,65 However, the level of evidence regarding the therapeutic efficacy of subthreshold laser is still low, and further prospective trials are needed.
Photobiomodulation is another promising non-pharmacological therapeutic strategy, based on administering low-energy light in the range of the near-infrared spectrum using a laser of a light-emitting diode. Again, the aim is to improve RPE metabolic activity and slow AMD progression.66 Some studies have reported positive effects of photobiomodulation on intermediate AMD. LIGHTSITE II was a prospective clinical trial, completed in 2021, that assessed the efficacy of photobiomodulation in dry AMD (ClinicalTrials.gov NCT03878420). However, according to a Cochrane meta-analysis,43 the real efficacy of photobiomodulation is still unclear and further studies are warranted to draw definite conclusions.
Conclusions
Today, several therapeutic options are available for managing wet AMD. In contrast, dry AMD and GA are still waiting for effective treatments, and nutraceutical approaches remain the only option. Classes of drugs other than anti-VEGF molecules are under investigation to improve the long-term management of patients with AMD, as are PDS and gene therapy approaches. With a growing incidence of wet and dry AMD creating an unsustainable burden of treatment for public health systems, future efforts should be dedicated to developing more efficient therapies that can optimize the use of available resources and further improve the management and long-term quality of life of patients with AMD.