Age-related macular degeneration (AMD) is characterized by age-associated thinning of the macula and formation of drusen. It has substantial global prevalence, especially in the older population, and it is the primary cause of permanent loss of vision in individuals 50 years and older in developd countries.1 Degeneration in the early and intermediate stages is known as atrophic AMD, whereas late-stage degeneration can progress to geographic atrophy (GA) or neovascular AMD (nAMD). nAMD, or wet AMD, is characterized by the growth of a new abnormal vasculature extending from the choroid referred to as choroidal neovascularization (CNV). Abnormal vasculature can also originate from the retina, resulting in the downgrowth of retinal circulation, which is known as type 3 macular neovascularization. CNV progression in AMD can lead to photoreceptor degeneration, which is the disruption of the retinal pigment epithelium (RPE), and macular damage.2 In nAMD, CNV leaks fluid, lipid and blood in the outer retina, which can lead to severe, irreversible vision loss if left untreated.
Atrophic AMD, also known as non-exudative AMD, has the potential to progress to an advanced stage of degeneration known as GA.2 GA is characterized by progressive, irreversible loss of vision due to the demarcated atrophy of the macular photoreceptors, choriocapillaris and RPE cells.3 GA enlargement can progress to a state of irreversibility, furthering disease progression.3 Various risk factors are inherently associated with GA, including age, genetics, smoking status and concurrent health conditions.2
Currently, no effective treatments for GA have been approved by the US Food and Drug Administration (FDA). However, avacincaptad pegol (Zimura®; IVERIC bio Inc, New York, NY, USA), a potent complement C5 inhibitor and potential therapeutic to treat GA, has been granted a Fast Track designation by the US FDA.4 The Fast Track designation facilitates the development and expedited review of drugs that have the potential to treat fatal medical conditions or diseases.4 Alongside avacincaptad pegol, there are several other ongoing investigations for potential therapeutics for GA secondary to AMD, such as APL-2 (A Phase II, Multicenter, Randomized, Single-Masked, Sham-Controlled Study of Safety, Tolerability and Evidence of Activity of Intravitreal APL-2 Therapy in Patients With Geographic Atrophy [GA]; ClinicalTrials.gov identifier: NCT02503332).5 If the results of GATHER2 (A Phase 3 Multicenter, Randomized, Double Masked, Sham- Controlled Clinical Trial to Assess the Safety and Efficacy of Intravitreal Administration of Zimura [Complement C5 Inhibitor] in Patients With Geographic Atrophy Secondary to Age-Related Macular Degeneration; ClinicalTrials.gov identifier: NCT04435366)6 confirm the data found in the previous avacincaptad pegol clinical trial, GATHER1 (A Phase 2/3 Randomized, Double-Masked, Controlled Trial to Assess the Safety and Efficacy of Intravitreous Administration of Zimura™ [Anti-C5 Aptamer] in Subjects With Geographic Atrophy Secondary to Dry Age-Related Macular Degeneration; ClinicalTrials.gov identifier: NCT02686658),7 avacincaptad pegol may advance as a potential therapeutic agent for GA. In this article, we review the potential efficacy and safety of a potent complement C5 inhibitor, avacincaptad pegol, as a treatment option in patients with GA.
Complement cascade in geographic atrophy
The complement cascade, or complement system, consists of many protein fragments that interact in a cascade, resulting in a humoral immune response. This system can be activated via three different pathways: classical, mannose-binding lectin, or alternative.8 The classical and lectin pathways are activated upon the recognition of specific antibody–antigen and mannose complexes binding on pathogens, respectively.9 The alternative pathway is constitutively active at relatively low levels due to spontaneous C3 hydrolysis but can also be amplified via a feedback loop.10 Regardless of which pathway is activated, all three pathways involved in the complement cascade intersect by forming each pathway’s respective C3 convertase.9
Subsequently, C3 convertase cleaves C3, resulting in C3a and C3b, which play a role in inflammation response and opsonization, respectively.8 C3b can be used to amplify the alternative pathway’s activity via a feedback loop, as it is a primary component in the alternative pathway’s C3 convertase structure (C3bBb), which may result in a majority of the pathway’s overall activity.9 C3b is also part of the formation of C5 convertase, the subsequent step in the cascade. C5, another major protein within the cascade, is split into two components by C5 convertase: C5a and C5b.8 C5a induces an inflammatory response, whereas C5b is a component of the terminal membrane attack complex (MAC) responsible for cell death.3
The complement system functions to keep tissue healthy but can also allow for excessive apoptosis when unregulated. It is hypothesized that unregulated activation of the alternative pathway contributes to the pathogenesis of AMD, as excessive MAC in choriocapillaris and the RPE can lead to atrophy.11,12 Under normal conditions, the alternative pathway is tightly regulated by proteins, including factor H, factor H-like protein 1 and properdin.13 However, it has been suggested that about 50% of people afflicted with dry AMD have mutations in genes encoding for complement regulatory proteins.14 It has also been suggested that the expression and localization of proteins involved in the complement cascade change with age.12 For example, elevated levels of activated complement proteins have been found in people afflicted with AMD and GA.9 The MAC, which mediates cell lysis, can be found at elevated levels on the choriocapillaris and RPE cells of those diagnosed with AMD, further demonstrating how excessive complement is implicated in deteriorating retinal health.12
Avacincaptad pegol is a pegylated RNA aptamer that specifically binds to C5, preventing the cleavage of C5 and, thus, the final steps of the complement system from occurring.15 Avacincaptad pegol is administered intravitreally to target the complement system within the eye, thus reducing the level of uncontrolled MAC formation and subsequent RPE disruption. The objective of this investigational therapeutic is to reduce the level of unnecessary RPE and photoreceptor atrophy, slowing the progression of GA.
Supporting clinical data
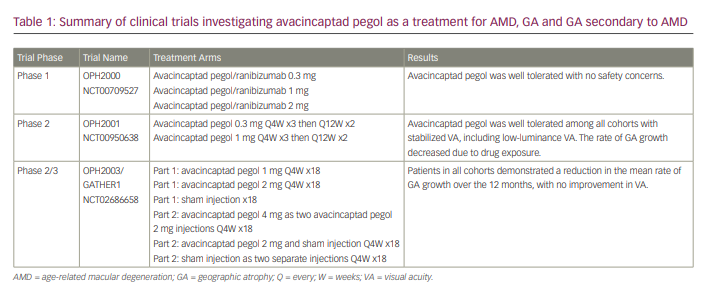
The safety and efficacy of avacincaptad pegol in patients with nAMD have previously been studied. The phase 1 ascending dose trial, OPH2000, evaluated the safety, dosing and efficacy of intravitreal (IVT) injections of a combination of avacincaptad pegol and ranibizumab (Lucentis®) 0.5 mg in 43 patients with nAMD (A Phase 1 Ascending Dose And Parallel Group Trial To Establish The Safety, Tolerability AND Pharmacokinetic Profile Of Multiple Intravitreous Injections Of ARC1905 [ANTI-C5 APTAMER] Given Either In Combination Therapy With Multiple Doses Of Lucentis® 0.5 mg/Eye, Or With One Induction Dose Of Lucentis® 0.5 mg/Eye In Subjects With Neovascular Age-Related Macular Degeneration; ClinicalTrials.gov identifier: NCT00709527).16 Patients with subfoveal nAMD were randomized in three cohorts by avacincaptad pegol dose: 0.3 mg, 1 mg and 2 mg. They were given six monthly IVT injections. The primary outcome was evaluated by changes in visual acuity (VA), measured by the Early Treatment Diabetic Retinopathy Study letters from baseline to week 24.17 During the 6-month trial, dose-limiting toxicity was not noted. At each cohort of avacincaptad pegol (0.3 mg, 1 mg and 2 mg), 46%, 47% and 60% of patients gained three or more lines of VA, respectively.18 Optical coherence tomography identified that the mean change in center point thickness was -150 µm.18 The combination of avacincaptad pegol and ranibizumab 0.5 mg was well tolerated, with no reported adverse events (AEs) related to the administration of the therapeutic agent at varying dose levels and ranibizumab 0.5 mg.17
A phase 1/2a trial, OPH2001, evaluated the efficacy and tolerability of avacincaptad pegol in patients with GA over 36 weeks (A Phase I Study of ARC1905 [Anti-C5 Aptamer] in Subjects With Dry Age-related Macular Degeneration; ClinicalTrials.gov Identifier: NCT00950638).19 A total of 47 patients were enrolled in this study and randomized into two dose arms: a 0.3 mg avacincaptad pegol arm and a 1 mg avacincaptad pegol arm. IVT injections of avacincaptad pegol were administered to both dose arms at day 0, week 4 and week 8, with subsequent injections at weeks 24 and 36.17 Follow-up visits were conducted at weeks 16 and 48. Various safety assessments were conducted, including VA assessments and intraocular pressure changes. The results of the study suggest that the 1 mg dose of avacincaptad pegol demonstrated a greater reduction in the mean growth of GA than the 0.3 mg dose. In the study eyes, the incidence of conversion to nAMD was not observed throughout the duration of the study. Notably, avacincaptad pegol was well tolerated, with no AEs reported in relation to it.17
GATHER1
The safety and efficacy of avacincaptad pegol were tested in the GATHER1 phase 2/3, double-masked clinical trial, also identified as OPH2003 (A Phase 2/3 Randomized, Double-Masked, Controlled Trial to Assess the Safety and Efficacy of Intravitreous Administration of Zimura™ [Anti-C5 Aptamer] in Subjects With Geographic Atrophy Secondary to Dry Age-Related Macular Degeneration; ClinicalTrials.gov Identifier: NCT02686658).7 A total of 286 subjects with GA secondary to AMD were randomized to receive either a sham injection, 2 mg or 4 mg of avacincaptad pegol monthly for 18 months. Both treatment arms resulted in a significant reduction in GA progression over the course of the trial (2 mg arm: p=0.0072; 4 mg arm: p=0.0051).2 GATHER1 showed a reduction in GA progression as early as 6 months of the 2 mg or 4 mg monthly injections of avacincaptad pegol. The mean rate of change in GA growth was evaluated via fundus autofluorescence (FAF) to determine the lesion size as a surrogate marker for vision loss at baseline, month 6, and month 12 as the primary efficacy endpoint (Figure 1).
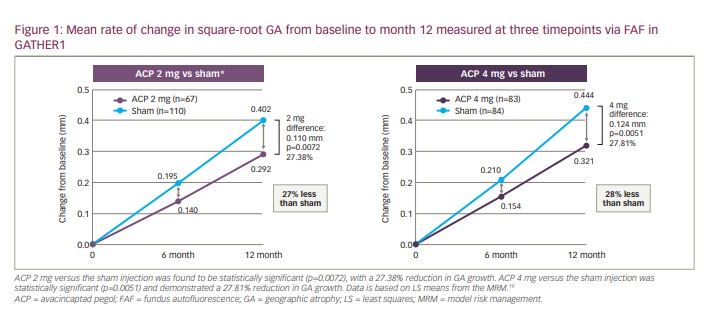
Over the 18-month study period, reduction in the mean rate of GA growth was 28.11% for the avacincaptad pegol 2 mg group, and 29.97% for the avacincaptad pegol 4 mg group.15 The effects of avacincaptad pegol are maintained over the course of the entire study, indicating the potential for sustained treatment. Over 18 months, 63.6% of patients in the combined avacincaptad pegol groups and 40.9% of patients in the combined sham injection group were reported to have at least one ocular treatment-emergent AE in the study eye.15
The increasing incidence of GA within the global population has highlighted the need for a therapeutic agent to limit further loss of vision in diseased eyes. Current and previous clinical trials have demonstrated the structural benefit of avacincaptad pegol as a monotherapeutic agent, as well as in combination with other therapeutics (Table 1).
GATHER2
Avacincaptad pegol is currently being evaluated in a second confirmatory phase 3 pivotal clinical trial for GA: GATHER2. This trial is a multicenter, randomized, double-masked study that aims to investigate the efficacy of IVT administration of avacincaptad pegol 2 mg in patients with GA secondary to AMD. A total of 448 patients were enrolled in the study, with enrollment completed at the end of June 2021.3 Patients are randomized to receive monthly injections of either avacincaptad pegol 2 mg or a sham injection for 12 months. At month 12, avacincaptad pegol arm patients are re-randomized into either continuing with the monthly avacincaptad pegol injections or receiving avacincaptad pegol injections every other month from month 12–23. The sham arm patients continue with monthly injections from month 12–23. The primary efficacy endpoint is evaluated by the mean rate of GA growth over three time points – baseline, month 6 and month 12 – via FAF photography. The primary results of the analysis are expected in the second half of 2022.
Conclusion
Recent preliminary data investigating the efficacy, safety, and dosing of avacincaptad pegol IVT injections indicate that avacincaptad pegol may be advanced as a further therapeutic agent available to treat GA. Hyper-activation of the complement system can facilitate the onset of GA, with associated peripheral or central vision loss. Avacincaptad pegol has demonstrated its effectiveness and role as a potent complement C5 inhibitor in various clinical trials, with an associated reduction in the mean growth rate of GA secondary to AMD. With promising results demonstrated in the GATHER1 trial, avacincaptad pegol is on track for expedited registration as a therapeutic agent for GA. The results from the completion of the GATHER2 trial in 2022 will have to correspond to favourable outcomes for avacincaptad pegol to ensure that the submission to the health authorities is of significant value to the patients.







