Triamcinolone acetonide (TA) is a synthetic steroid of the glucocorticoid family with a fluorine in the ninth position.1 It is commercially available as an ester and represents one of the most commonly used steroid agents for the treatment of several retinal conditions.2
Triamcinolone acetonide (TA) is a synthetic steroid of the glucocorticoid family with a fluorine in the ninth position.1 It is commercially available as an ester and represents one of the most commonly used steroid agents for the treatment of several retinal conditions.2
TA has an anti-inflammatory potency five times higher than hydrocortisone with a 10th of the sodium-retaining potency. It appears as a white- to cream-colored crystalline powder and it is practically insoluble in water and very soluble in alcohol.3 The decreased water solubility accounts for its prolonged duration of action. It has been observed that adequate concentrations of TA could provide therapeutic effects for approximately three months after 4 mg intravitreal TA injection.4 A maximum effect duration of 140 days has been suggested.5,6
Mechanism
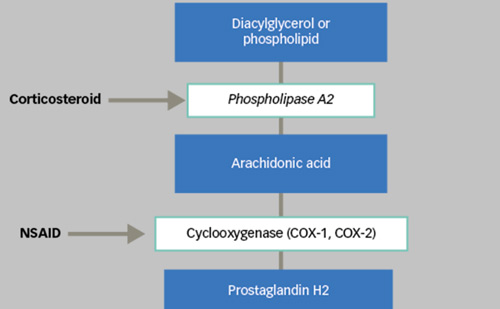
TA has been shown to inhibit the inflammatory response, thereby reducing oedema formation, leukocyte migration, capillary dilatation and fibroblast proliferation. Steroids are thought to act by the induction of proteins called lipocortins, in particular phospholipase A2. These proteins reduce leukocyte chemotaxis, control biosynthesis and inhibit the release of arachidonic acid from the phospholipid membrane, which is one of the most important common precursors of potent inflammatory cell mediators such as prostaglandins and leukotrienes.8 The anti-inflammatory, angiostatic and anti-permeability proprieties of corticosteroids seem also to be related to the regulation of gene expression components. This regulation influences the expression of vascular endothelial growth factor (VEGF), inhibits pro-inflammatory genes such as tumour necrosis factor-alpha (TNF-α) and other inflammatory chemokines, and induces the expression of anti-inflammatory factors such as pigment epithelium-derived factor (PEDF).9–11 Some studies show that TA, at therapeutic concentrations, significantly inhibits the expression of TNF-α, interleukin 1-beta (IL-1β), thromboxane B2 (TxB2) and leukotriene B4 (LTB4), in a dose-dependent manner.12 Additionally, TA seems to reduce the expression of matrix metalloproteinases (MMPs) and to downregulate intercellular adhesion molecule 1 (ICAM-1) on choroidal endothelial cells.13
Efficacy of Intravitreal Triamcinolone Acetonide
Based on several studies, intravitreal administration of TA has provided promising results for the treatment of disorders associated with an abnormal endothelial cell proliferation and conditions complicated by intra-retinal and subretinal fluid accumulation. The anti-inflammatory, angiostatic and anti-permeability properties of TA have gained interest in chronic retinal diseases, such as diabetic macular oedema (DMO).14–18 DMO is the leading cause of vision loss in the working-age population and it occurs as an increased accumulation of fluid within the intra-retinal layers of the macula as a result of retinal microvascular changes and disruption of the blood–retinal barrier. The rationale for using a steroidal drug for the treatment of oedematous and proliferative diseases is that abnormal proliferation of cells is often associated with and trigged by inflammation and intra-retinal accumulation of fluid is usually accompanied by a blood–retinal barrier dysfunction that can be restored with steroid therapy.19 Intravitreal TA has been widely studied in many randomised clinical trials on DMO demonstrating significant improvements both in morphological and functional outcomes.20–23 Focal and grid laser photocoagulation has been considered the standard of care for the treatment of DMO for many years.24,25 However, a substantial group of patients are unresponsive to laser therapy and fail to improve after photocoagulation. It has been reported that three years after initial grid treatment visual acuity improved in 14.5 % of eyes, did not change in 60.9 % and decreased in 24.6 % of patients with DMO.26 Therefore, TA has been tested for the treatment of DMO, either naive or diffuse and refractory to laser therapy. In most cases, TA has been administered intravitreally. However, other delivery routes have been tested, such as sub-Tenon, juxtascleral and sub-conjuntival administration. The current commercial preparations of TA include products that received dermatologic and orthopaedic indications and are considered off-label for the intraocular use, products registered as devices for assisting the visualisation of the vitreous during vitreoretinal procedures and products that are registered for intraocular use in uveitis and other ocular inflammatory conditions. Kenalog-40 (40 mg/ml, Bristol-Myers Squibb, NJ) is the most common intraocular steroid and has been widely used as intravitreal injections since 2004 for the treatment of several retinal diseases. This formulation is US Food and Drug Administration (FDA)-approved only for intramuscular and intra-articular use and is currently employed off-label for intraocular injections. Trivaris™ (80 mg/ml, Allergan Inc., Irvine, CA) and Triesence® (40 mg/ml, Alcon Inc., Fort Worth, TX) are preservative-free brands of TA recently FDA approved for ophthalmic use in the treatment of sympathetic ophthalmia, temporal arteritis, uveitis and other ocular inflammatory diseases unresponsive to topical corticosteroids. Vitreal S (Sooft s.p.a., Fermo, Italy) is a medical device used in endocular surgery to stain the vitreous during vitrectomy and it is not registered as drug for intraocular use. There are some issues regarding the formulation of TA used for intraocular administration. A previous phase-contrast microscopy study showed a notable difference of crystal size depending upon the drug formulation.27 Very large and irregular crystals, with a significant heterogeneity in crystal size, were occasionally found in the off-label commercially available, benzyl-alcohol-preserved TA, whereas the crystals of a preservative-free in-label commercially available TA suspension appeared to be relatively uniform in size. These morphologic aspects may have a significant impact on the half-life of the drug both in vivo and in vitro. This hypothesis is based on the fact that smaller crystals have a superior surface-area-to-volume ratio, allowing them to be dissolved more rapidly. The formulations containing crystals that widely vary in size and, thus, including larger crystals may theoretically generate a wider time–drug concentration curve because of their slower dissolution rate.27–29
A carefully designed prospective randomised trial conducted by the Diabetic Retinopathy Clinical Research Network (DRCR.net) investigated the efficacy and safety of 1 mg and 4 mg doses of preservative-free intravitreal TA in comparison with focal or grid laser photocoagulation.30 In the DRCR.net study, 840 study eyes with DMO were randomised to either focal or grid laser photocoagulation (n=330), 1 mg TA (n=256) or 4 mg TA (n=254). At year three, the mean change in the visual acuity from baseline was +5 letters in the laser group and 0 letters in both the TA groups. For the three two-group comparisons, mean difference adjusted for baseline visual acuity and prior macular photocoagulation and 95 % confidence interval (CI) were as follows: +5.6 (95 % CI, +0.8 to +10.4) for laser versus 1 mg TA groups; +4.7 (95 % CI, 0.0 to +9.5) for laser versus 4 mg TA groups; and -0.8 (95 % CI, -6.0 to +4.3) for 1 mg TA versus 4 mg TA groups. A worsening of visual acuity of three or more lines occurred in 8 %, 17 % and 16% of eyes, respectively, and an improvement in visual acuity by three or more lines occurred in 26 %, 20 % and 21 % of eyes, respectively. Mean (±SD) reductions in central macular thickness were 175±149 μm in the laser group, 124±184 μm in the 1 mg TA group and 126±159 μm in the 4 mg TA group. The mean number of treatments at the end of the follow-up was 3.1 for the laser group, 4.2 for the 1 mg and 4.1 for the 4 mg TA groups. At the four-month visit, mean visual acuity improvement was higher in the 4 mg TA group (4±12 letters improvement) than in either the laser group (0±13 letters change) or the 1 mg TA group (0±13 letters change). By 12 months, there were no significant differences among groups in mean visual acuity. Therefore, in this study, photocoagulation was shown to be more effective over time and had fewer side effects than TA. This was considered in support of focal/grid photocoagulation. However, it must be noted that during the 36 months of follow-up, patients received only four treatments with intravitreal TA, which is a low reinjection rate based on common experience and pharmacokinetic (PK) data. Recently, a new, large, randomised DRCR.net study investigated the efficacy of intravitreal TA in combination with laser photocoagulation in comparison with intravitreal ranibizumab with prompt or deferred laser photocoagulation or laser photocoagulation alone. At two-year visit, compared with the sham + prompt laser group, the mean change in the visual acuity letter score from baseline was 3.7 letters greater in the ranibizumab + prompt laser group (p=0.03), 5.8 letters greater in the ranibizumab + deferred laser group (p<0.01) and 1.5 letters worse in the TA + prompt laser group (p=0.35). A worsening of visual acuity of three or more lines occurred in 10 %, 4 %, 2 % and 13 % of eyes, respectively, and an improvement in visual acuity by three or more lines occurred in 18 %, 29 %, 28 % and 22 % of eyes, respectively. Compared with the sham + prompt laser group, the mean change in central macular thickness from baseline was 31 μm worse in the ranibizumab + prompt laser group (p=0.03), 28 μm worse in the ranibizumab + deferred laser group (p=0.01) and 10 μm worse in the TA + prompt laser group (p=0.37). These results showed that intravitreal ranibizumab with prompt or deferred laser is more effective than prompt laser alone or intravitreal TA combined with laser for the treatment of diabetic macular oedema involving the central macula. Among the eyes that were pseudophakic at baseline, the mean change in visual acuity letter score from baseline to the two-year visit was 1.6 letters greater in the TA + prompt laser group compared with the sham + prompt laser group and was similar to difference in outcomes between the ranibizumab + prompt laser group (+0.5 letters) and the ranibizumab + deferred laser group (+3.5 letters) compared with the sham + prompt laser group. Cataract surgery was required in 12 % of phakic eyes in the sham + prompt laser and in the ranibizumab + prompt laser groups, in 13 % of phakic eyes in the ranibizumab + deferred laser group and in 55 % of patients of the TA + laser group. An intraocular pressure (IOP)-lowering medication was required in 5 % of eyes in the sham + prompt laser and ranibizumab + prompt laser groups, in 3 % of eyes in the ranibizumab + deferred laser group and in 28 % of patients of the TA + laser group.31 Other studies demonstrated promising results of combination therapy with intravitreal injection of TA and laser photocoagulation for the treatment of proliferative diabetic retinopathy (PDR) with clinically significant macular oedema (CSMO).32–36 In a 12-month randomised clinical trial conducted by Maia et al., 44 eyes with PDR and CSMO were enrolled and randomised to treatment with combined 4 mg of intravitreal TA and laser photocoagulation (n=22) or to laser photocoagulation alone (n=22). Mean best corrected visual acuity (BCVA) improved significantly (p<0.001) in the TA and laser group compared with the laser alone group at all study follow-up visits. An improvement of two or more Early Treatment Diabetic Retinopathy Study (ETDRS) lines was observed in 63.1 % and 10.5 % of eyes, respectively (p<0.001). A significant decrease in mean central macular thickness occurred in the TA and laser group when compared with the laser alone group at all study follow-up intervals (p<0.001). At 12 months, mean (±SD) reductions in central macular thickness were 123±68 μm and 65±51 μm, respectively (p<0.001).37 Several other studies reported favourable results of intravitreal TA in refractory DMO.23,38–40 In a six-month prospective, placebo-controlled, randomised clinical trial conducted by Jonas et al., 40 eyes with persistent DMO were enrolled and randomised to treatment with 20 mg TA (n=28) or to placebo injection (n=12). Visual acuity increased significantly (p<0.001) in the TA group by 3.4 ETDRS lines. In the placebo group, visual acuity did not change significantly (p=0.07) during the six months. At the end of follow-up period, 48 % in the TA group improved by at least two ETDRS lines compared with 0 % eyes in the placebo group.23 Recently, Gillies et al. reported the longest-term data available concerning the outcomes of intravitreal injection of TA. This was a five-year prospective, double-masked, randomised clinical trial of 4 mg dose of preservative-free intravitreal TA in comparison with placebo. In this study, 67 study eyes with refractory DMO were randomised to receive 4 mg TA (n=33) or placebo (n=34). At five years, an improvement in visual acuity of three or more lines occurred in 42 % of eyes in the TA group and 32 % of eyes in the placebo group (p=0.4). A worsening of visual acuity by three or more lines occurred in 18 % and 24 % of eyes, respectively (p=0.88). Mean (±SD) reductions in central macular thickness were 100±79 μm in the TA group and 184±29 μm in the placebo group (p=0.45). After five years the difference in visual acuity between the two groups was not statistically significant and there was no difference in mean central macular thickness reduction between two groups.39 Moreover, this study showed that, in the long term, a two-year delay in the beginning of intravitreal TA treatment did not seem to adversely affect outcomes in eyes affected with refractory DMO.
Dosage
The appropriate dose of intravitreal TA remains a subject of debate. Both Hauser et al. and Audren et al. showed that the use of a 4 mg dose of intravitreal TA does not have enough advantages over the lower 1 mg or 2 mg dose.40,41 However, Lam et al. published a comparison between 4 mg and 8 mg doses and showed that the higher dose had a more sustained effect on both visual acuity and central macular thickness, although with a trend to more ocular complications.42 By using a dose of about 20 mg of TA, the increase in visual acuity was most marked during the first three and six months after injection and was observable for a period of about six to nine months. Differently, by using a dose of 4 mg, the duration in the reduction of macular thickness as measured by optical coherence tomography (OCT) was less than six months.43
Other Routes of Administration
While intravitreal TA administration has been shown useful to reduce the macular oedema and to improve or at least stabilise visual acuity, these effects are often transient and associated with several adverse events. Therefore, emerging pharmacological approaches are being evaluated for the treatment of DMO, including extraocular delivery routes and intravitreal steroid-releasing implants. Geroski et al. reported that therapeutic doses of TA could reach the posterior segment via transscleral absorption with periocular administration.44 Thus, other routes of TA administration, such as sub-conjunctival, sub-Tenon and posterior juxtascleral infusions, have been considered. The commonly reported advantages of periocular administration of TA versus intravitreal injection include a lower risk of IOP elevation and endophthalmitis. However, peribulbar injections of TA seem to result in lower morphological and functional outcomes as compared with those reported with the use of intravitreal TA.45–50 Recently, a 12-month interventional case report demonstrated that posterior juxtascleral infusion of a viscoelastic formulation of TA is an effective treatment for diffuse DMO unresponsive to laser photocoagulation.51 Morphological results indicated that macular thickness changed significantly (p<0.001) after 12 months of follow-up. Serial comparison between baseline and post-treatment values demonstrated that the decrease in the mean central foveal thickness (CFT) was significant at each follow-up time (p<0.0005). One week after TA infusion, mean reduction in CFT was 134 μm and the effect duration reaches approximately six–nine months. One infusion only was given in 54.5 % of eyes. A decrease in macular thickening of 50 % or more was present in half of the eyes at the end of the 12 months of follow-up. Moreover, BCVA results changed significantly (p<0.006). Serial comparison between baseline and post-treatment values demonstrated that the improvement in mean BCVA was significant one, three, nine and 12 months after treatment (p<0.008). At the end of the study, the mean improvement in BCVA was 0.15 logMAR. In 63 % of eyes, the improvement was at least one ETDRS line, and in 27.3 % cases it was greater than three ETDRS lines. A BCVA reduction of more than three lines was noted in four cases during the follow-up time. To provide long-term drug delivery to the macular region and to limit the frequency of repeated intraocular TA injections, several intravitreal steroid-releasing therapies are being proposed for the treatment of DMO. A controlled-release microsphere system for TA has been recently reported. A single intravitreal injection of 1 mg TA in a controlled-release poly(lactide-co-glycolide) (PLGA) microsphere system (intravitreal bioerudivel sustained-release triamcinolone microspheres system; RETAAC system) has been compared with a single intravitreal injection of 4 mg TA in patients with DMO unresponsive to laser photocoagulation. Both the safety and the anatomic and functional outcomes were evaluated. RETAAC-treated eyes showed marked decrease of retinal thickness as well as improved visual acuity after 12 months of follow-up. This study demonstrated a superior long-term pharmacologic performance compared with TA-injected eyes. No drug- or procedure-related side effects were observed in this study.52 Another promising approach seems to be represented by Verisome® technology. Verisome is a sustained-release drug delivery system that can be injected into the eye as a liquid via a standard 30-gauge needle. The biodegradable vehicle provides controlled, extended drug release over a titratable period of up to one year. The liquid-gel formulation was designed to deliver TA for up to one year via a single intravitreal injection to treat patients with macular oedema associated with retinal vein occlusion.53 The results of the clinical trial confirmed the expected safety and efficacy characteristics and the controlled-release attributes of the technology. Recently, Verisome technology has been proposed to treat patients affected with DMO.54 Moreover, new biodegradable and non-biodegradable steroid delivery systems are being evaluated for long-term efficacy in chronic diseases, such as DMO. These include dexamethasone (Ozurdex®, Allergan Inc., Irvine, CA) and fluocinolone acetonide implants (Iluvien®, Alimera Sciences, Alpharetta, GA), both of which have an approximately five-fold increase in corticosteroid potency.55,56
Safety Profile
The safety evaluation of the administration of intravitreal TA has been investigated by several randomised controlled clinical trials. Potential complications of intravitreal steroid treatment are divided into steroid-related and injection-related adverse effects. Steroid-related side effects most commonly include the increase of IOP and cataract formation. Injection-related side effects include endophthalmitis, pseudoendophthalmitis, rhegmatogenous retinal detachment and toxic effects.
Post-injection Infectious Endophthalmitis
Infectious endophthalmitis is one of the most serious complications of intravitreal injection of TA, with the reported risk per injection ranging from 0.1 % to 1.6 %.57 Many studies suggest that this relatively high rate of infectious endophthalmitis may be attributed to the techniques used for injection. If the injection is performed under sterile conditions, the risk of an infection may be lower.58–65 Patients with infectious endophthalmitis after intravitreal TA usually present without pain, which is uncommon for infectious endophthalmitis in eyes without intraocular steroids.66
Post-injection Pseudoendophthalmitis
Several studies have described non-infectious endophthalmitis after intravitreal injection of TA.67,68 Post-injection pseudoendophthalmitis is present if TA crystals are washed from the vitreous cavity into the anterior chamber and settle down in the inferior anterior chamber angle mimicking hypopyon. According to reports, this complication occurs in 0.2–6.7 % of eyes following treatment. TA crystals in the anterior chamber usually disappear spontaneously and may not need to be removed. There have been no reports so far showing corneal endothelial damage or damage to the trabecular meshwork by the crystals.69
Steroid-induced Ocular Hypertension
A number of reports have described IOP elevation as the most common adverse event of intravitreal TA.70–82 Mild to moderate IOP elevation was seen in 28–42 % of patients, typically within the first three months following injection. This condition is usually controlled with topical agents alone. About 1 % of patients require surgical treatment. Selective laser trabeculoplasty (SLT) is a treatment alternative or adjunct to medical therapy. Comparing studies using different doses of TA for intravitreal injection suggests that the risk of IOP rise appears to be higher due to the prolonged elevated concentrations that are achieved intraocularly. If further studies confirm the assumption that the frequency of secondary ocular hypertension after an intravitreal TA injection may not markedly depend on the dose used, one may assume that even relatively low TA doses are already high enough to occupy all steroid receptors. Some authors suggest that pre-medication with topical steroids may be useful to identify possible steroid responders and excluding them from intravitreal TA treatment may lower the incidence of IOP elevation. 83–85
Steroid-induced Cataract
Steroid-induced cataract is a common side effect of intravitreal TA.77,86–88 A recent study reported that, in the elderly population, intravitreal high-dose injections of TA led to clinically significant cataract with eventual cataract surgery in about 15–20 % of eyes within about one year of the intravitreal injection.86 Gillies et al. concluded that eyes with an elevation of IOP after intravitreal TA have a very high risk of rapidly experiencing posterior subcapsular lens opacification.87 This strong association suggests a similar mechanism responsible for the development of steroid-induced posterior subcapsular cataract and for the elevation of IOP. A study suggested that a single intravitreal TA induces posterior subcapsular cataract development, whereas multiple injections result in all-layer cataract progression.88
Rhegmatogenous Retinal Detachment
A potential complication of the intravitreal TA injection may be a rhegmatogenous retinal detachment.89 Triamcinolone acetonide, injected into the vitreous cavity, leads to a change in the structure of the vitreous body and the abnormal vitreous may exert traction on the retina. In particular, this is supposed for the inferior midperipheral area of the vitreo-retinal interface where the TA crystals remain in the preretinal vitreal cortex; for superior midperipheral and peripheral regions where a vitreous traction might be induced by the weight of the TA crystals settled at six o’clock; and for the far periphery of the fundus where the vitreous, incarcerated into the injection site, causes retinal traction.
Toxic Effects
Previous studies in rabbits found that preservatives in the vehicle for suspension of crystalline steroids, rather than steroid itself, could be toxic to the rabbit retina and lens and that the vehicle is not totally responsible for the toxicity, but may initiate TA-dependent toxicity.90,91 Direct toxic effects of TA on the retina and optic nerve have not yet been observed, independently of the dose used.92 TA has been shown to be toxic to retinal pigment epithelial cells in vitro,93 whereas ex vivo94 and in vivo95 studies have failed to show any significant toxicity to the retina. Because TA is a heavy depot formulated suspension, it settles in the inferior vitreous cavity. Whereas there is certainly distribution of the drug throughout the vitreous cavity due to diffusion and constant eye movements, it is possible that the drug does not distribute equally in the vitreous cavity and that the concentration of the drug at the macula is different (presumably lower) than in the inferior retinal periphery.96 Yeung et al. reported a possible cytotoxic effect of TA, causing a significant reduction in cell numbers throughout the whole range of concentrations when retinal pigment epithelium cells were exposed to it for more than one day.97 Compared with dexamethasone and hydrocortisone, TA showed the higher relative toxicity.
Safety of Intravitreal TA Including High-dose Reinjections
In a case series study, Gilles et al. showed that side effects or complications may not occur more frequently after reinjections of TA than after a primary intravitreal high-dose injection.81 Moreover, the intravitreal high-dose reinjections may be tolerated by eyes within a mean follow-up of about 21 months after the first injection, or about 10 months after the last injection and the increase in IOP may not be more marked after a repeated injection than after the first injection.
Systemic Safety
In the randomised study from DRCR.net comparing laser photocoagulation with ranibizumab in combination with laser and intravitreal TA associated with laser, no evidence suggests that the administration of TA is associated with an increased risk of systemic adverse events, including stroke or cardiac events. Two-year incidence of non-fatal myocardial infarction was 3 % in the laser alone group, 1 % in the ranibizumab–laser group and 3 % in the TA–laser group. Any cardiovascular event, as defined by the Antiplatelet Trialists’ Collaboration (ATC), occurred in 12 % of the laser alone group, 5 % of the ranibizumab–laser group and 6 % of the TA–laser group.31
Conclusions
DMO remains the leading cause of visual impairment in the working-age population. Emerging pharmacological approaches are being evaluated to treat DMO unresponsive to laser therapy. Many retinal physicians have begun to routinely inject TA as a promising option for the treatment of refractory DMO, although in these cases the intravitreal administration of TA is not FDA approved and has been mostly used off-label. Intravitreal TA has been found to significantly increase the visual acuity and decrease central macular thickness in short-term follow-up. Despite a very favourable systemic safety profile, a significant proportion of patients experience a rise in IOP and cataract development following intravitreal TA injections. The incidence of severe ocular adverse events such as infectious endophthalmitis, pseudoendophthalmitis, retinal toxicity and rhegmatogenous retinal detachment remains on the low side. Recently, a combined strategy of intravitreal injection of TA and laser photocoagulation has been evaluated for the treatment of DMO.31 In this study laser photocoagulation appeared safer and more effective than the combination of intravitreal injection of TA and laser treatment. However, when considering patients that were pseudophakic at baseline, TA in combination with laser resulted in better visual outcomes than laser alone. The results of the combination of TA and laser were comparable to those obtained with the combination of laser and ranibizumab in this subgroup of patients after two years of follow-up.31 The rationale for combining laser photocoagulation and intravitreal TA lies in their synergistic mechanism of action and may offer the chance to reduce the number of intravitreal injections required and so decrease the rate of drug- and injection-related adverse events. However, several vision-threatening side effects have been reported as a result of thermal damage caused by laser procedure.98–100 In recent years advances in laser therapy of retinal diseases have been directed at reducing the unnecessary disruptive effect that laser photocoagulation produces in retinal tissues. Several studies have shown the efficacy of sub-threshold laser photocoagulation in treating DMO, producing fewer side effects than conventional laser treatment.101–105 Combining intravitreal TA and sub-threshold laser photocoagulation may be a promising option to obtain good and durable visual outcomes while reducing the side effects correlated to either the laser procedure or the drug.
Many questions still remain unanswered concerning the optimal dose of TA for intravitreal use and the side-effect profiles of various commercially available formulations of TA with and without preservatives. Thus, an optimal balance between efficacy and safety profile has yet to be completely determined. Novel steroid implants and anti-VEGF drugs are being evaluated alone or in combination as promising options in the emerging armamentarium for the treatment of DMO. ■