Photodynamic therapy (PDT) is a technique involving the use of a photosensitizing agent that, when activated by light of a specific wavelength, causes localized and selective tissue damage.1,2 Initially developed to treat tumor cells using tumor-localizing photosensitizing agents (e.g., Photofrin®, Pinnacle Biologics, Chicago, IL USA),1 ophthalmic PDT was developed in the 1990s to treat conditions such as choroidal neovascularization (CNV) associated with age-related macular degeneration (AMD).2 While several ophthalmic sensitizing agents were initially developed, verteporfin was shown to have the most promising overall efficacy, safety, and chemical characteristics (e.g., a longer absorption spectrum, thereby enabling activation further into the tissue, a lipophilic nature to support localization, and a short half-life to minimize the duration of skin photosensitivity).2–4 Here we review the mechanism of action, current applications, and supporting evidence for the use of verteporfin PDT (vPDT) in the treatment of various choroidal conditions, including CNV associated with AMD, central serous chorioretinopathy (CSC), polypoidal choroidal vasculopathy (PCV), choroidal hemangioma, and peripapillary CNV.
Verteporfin photodynamic therapy mechanism of action and standardized protocol
Verteporfin is transported in the plasma, primarily by low density lipoproteins, and was developed to preferentially accumulate in abnormal neovascular endothelial cells, due to an increased expression of low density lipoproteins receptors compared with normal choroidal and retinal vessels.2,5,6 Once photoactivated, verteporfin generates highly reactive, short-lived singlet oxygen and reactive oxygen radicals, causing selective and local damage to neovascular endothelium and choroidal vascular occlusion.2,5 This selective occlusion of choroidal neovasculature by vPDT causes minimal damage to the neurosensory retina and, therefore, does not induce loss of visual acuity (VA).7 This allows vPDT to be used in the large proportion of patients not eligible for more destructive procedures such as photocoagulation.7
The ‘standard’ vPDT protocol is a two-step procedure developed for use in patients with subfoveal CNV associated with AMD, and first used in the TAP (Treatment of Age-related macular degeneration with photodynamic Therapy) and VIP (Verteporfin In Photodynamic therapy) studies (Table 1).8–10 The procedure involves two main steps: (i) intravenous administration of verteporfin at a dose of 6 mg/m2 over 10 minutes at a rate of 3 mL/min, followed by a 5-minute wait;5,11 and (ii) photoactivation of the verteporfin using laser light of 689 nm at a recommended light dose of 50 J/cm2 per neovascular lesion at an intensity of 600 mW/cm2 over 83 seconds.5,11 The use of 689 nm red light ensures good penetration through melanin, blood, and fibrotic tissue and effective treatment of the choroidal vasculature.2 The vPDT laser delivery system also produces a circular spot of light, which is adjusted to exceed the greatest linear dimension of the target lesion, usually up to a maximum of 7,000 μm.2
While the standard vPDT protocol was shown to have a favorable safety profile in the TAP and VIP studies,8–10 as the photochemical reaction has a dose-dependent response, different protocols have since been developed to enhance the efficacy and/or safety of vPDT, usually by adjusting either the verteporfin dose administered (e.g. 3 mg/m2) or the laser fluence (e.g. 25 J/cm2).2 As a result of this adaptability, vPDT has potential applications across a range of chorioretinal conditions, which will be discussed here.
Several reviews published approximately a decade ago, indicated, at the time, that vPDT was the treatment of choice for common and, some less common, choroidal neovascular conditions.12–15 This was supported by numerous clinical studies that provided high-quality evidence. Since that time, the emergence of the anti-vascular endothelial growth factor (VEGF) agents, particularly ranibizumab, aflibercept, and bevacizumab, has changed the treatment landscape and these have now become the standard therapies.16 Nevertheless, vPDT remains a valuable treatment; some current recommendations state that poor or non-response to anti-VEGF treatments requires re-evaluation of diagnosis and, if necessary, a switch to alternative therapies including other anti-VEGF agents and/or vPDT.17
Choroidal neovascularization
AMD is a progressive maculopathy that typically occurs in patients >50 years and has two distinct classifications: ‘dry’ AMD, characterized by non-neovascular pathology with drusen or abnormalities of the retinal pigment epithelium (RPE); and ‘wet’ (neovascular) AMD, characterized by CNV.18,19 With the latter, neovascular proliferation from the choriocapillaris extends under the Bruch’s membrane and invades the space under the RPE, causing the leakage of serous fluid.18,20 This is associated with the development of fibrous tissues that replace the normal architecture of the outer retina, and often leads to RPE detachment and atrophy, hemorrhages, scarring, and severe and irreversible loss of central vision.18,19 Although neovascular AMD is less prevalent than dry AMD, with approximately 500,000 new cases of neovascular AMD occurring each year, it accounts for ~90% of all AMD cases that result in vision loss and it has been estimated that approximately 8 million older-age adults are at high risk of developing AMD in the US alone.18,21 AMD risk can be reduced by diet; the large-scale Age Related Eye Disease study (AREDS) reported that a nutritional supplement known as the AREDS formulation, which contained vitamin C, vitamin E, beta-carotene, zinc and copper, reduced the risk of AMD progression.22 However, beta carotene was associated with an increased risk of lung cancer in smokers. The subsequent AREDS2 reported that lutein and zeaxanthin could be used as a safe and effective alternative to beta carotene, but the addition of omega-3 fatty acids did not provide a statistically significant benefit in study participants.22 The ideal treatment for neovascular AMD would involve the destruction of the choroidal neovasculature while preserving the overlying retina.18 As such, vPDT provides a viable therapeutic approach to the treatment of this condition.
Following promising results in early phase I and phase II clinical trials of single vPDT treatments in CNV,3 multiple vPDT treatment strategies were investigated for the treatment of CNV associated with AMD.8,10 In 2001, the TAP study investigated the effectiveness of vPDT therapy in the treatment of predominantly classic CNV lesions in patients with AMD.8,10 At both 12 and 24 months following vPDT treatment, significantly more patients in the vPDT group had lost fewer than 15 letters (~3 lines) of VA compared with the placebo group (both p<0.001; Table 1).8,10 At 12 months, few ocular/systemic adverse events were associated with verteporfin treatment, compared with placebo (PDT with 5% dextrose in water), including transient visual disturbances (18% versus 12%), injection-site adverse events (13% versus 3%), transient photosensitivity reactions (3% versus 0%), and infusion-related low back pain (2% versus 0%).10 Few additional photosensitivity adverse reactions and injection site adverse events were associated with vPDT in the second year of follow-up (Table 1).8
However, in 2006, results from the MARINA and ANCHOR studies demonstrated the effectiveness of anti-VEGF therapy (ranibizumab) for the treatment of all types of CNV lesions in AMD, not only compared with placebo (sham injections), but also compared with vPDT therapy (Table 1).23–25 In both these studies ranibizumab was shown to stabilize VA for up to 2 years in the large majority of patients (≥90%), and even improve VA in ~30% of patients, with a favorable safety profile.23–25 Anti-VEGF therapy subsequently became the modality of choice for the treatment of neovascular AMD.
Since then, several studies have investigated the potential benefits of anti-VEGF and vPDT dual combination therapy (DENALI and MONT BLANC studies)26,27 and anti-VEGF, vPDT and dexamethasone triple therapy (RADICAL study),28 for the treatment of CNV associated with AMD (Table 1). In the DENALI study, although the non-inferiority (defined as a best-corrected visual acuity [BCVA] margin of ≤7 letters) of combined vPDT + ranibizumab regimens (standard or reduced fluence vPDT + 0.5 mg) to ranibizumab monotherapy (0.5 mg) was not demonstrated at 1 year, fewer ranibizumab re-treatments were required with vPDT combination therapy (5.1 and 5.7 versus 10.5 injections, with standard and reduced vPDT versus ranibizumab monotherapy, respectively).26 In contrast, the MONT BLANC study did demonstrate the non-inferiority (again, defined as a BCVA margin of ≤7 letters) of vPDT + ranibizumab combination therapy compared with ranibizumab monotherapy at 1 year, but with no significant benefit to the number of ranibizumab re-treatments.27
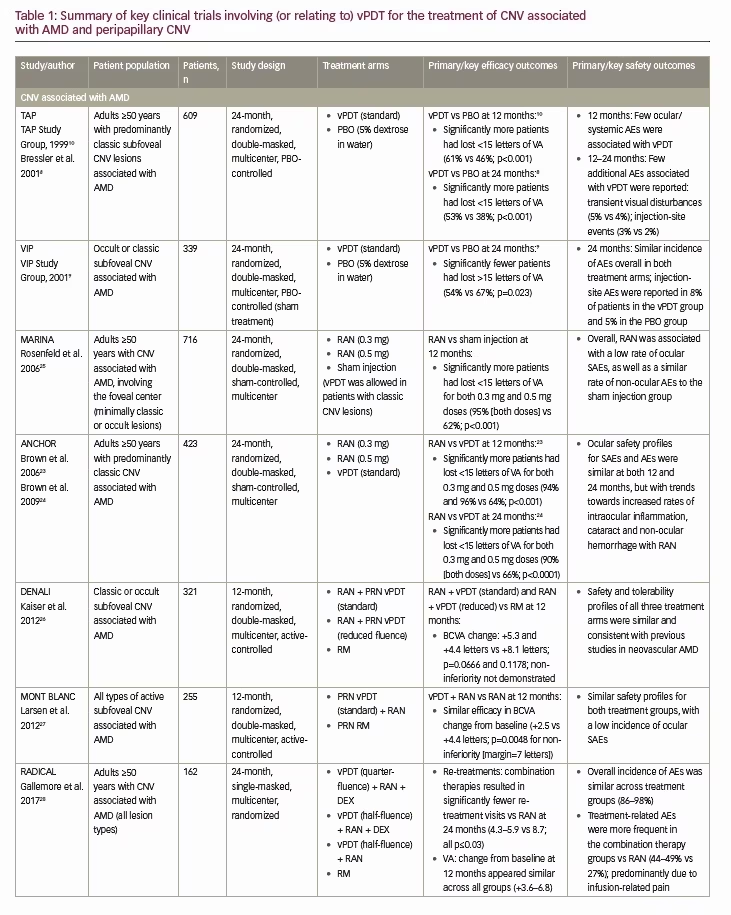
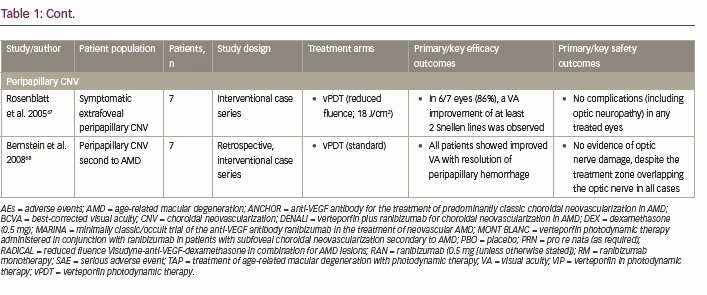
The RADICAL study also showed significantly fewer re-treatment visits with triple combination therapy (vPDT + ranibizumab + dexamethasone) compared with ranibizumab monotherapy for up to 2 years, with similar apparent improvements in VA between the treatment groups.28 Studies of vPDT in non-AMD-associated CNV present similar findings to those in neovascular AMD, with anti-VEGF therapy providing better visual outcomes (though requiring more re-treatments) than vPDT in myopic CNV for up to 1 year (RADIANCE study),29 better visual outcomes than vPDT for up to 2 years in idiopathic CNV,30 and clinical benefits in other causes of CNV including pathologic myopia, ocular histoplasmosis, and angioid streaks.31 Nevertheless, vPDT remains an important treatment in the chorioretinal armamentarium, not only as alternative therapy in patients with AMD-associated CNV who do not respond to anti-VEGF therapy (or are unable to tolerate the intravitreal injections),32 but potentially in alternative choroidal conditions with similar pathologies, such as CSC, CSC with CNV, PCV, choroidal hemangioma, and peripapillary CNV. It should be noted, that although vPDT is important in PVC and CSC, it has become less important and less used in the treatment of wet AMD.
Central serous chorioretinopathy
CSC is a disorder characterized by serous retinal detachment and/or RPE detachment, changes most often confined to the macula, and is associated with leakage of fluid through the RPE into the subretinal space.33 It is broadly classified into two categories: acute CSC, which tends to affect younger patients and generally resolves spontaneously within 1–4 months; and chronic or recurrent CSC, which involves frequent recurrences or chronic retinal detachment that can lead to RPE atrophy and permanent loss of visual function.33 Overall, CSC typically affects men (male:female ratio of 2.7:1 to 7:1) with an average age of 45–51 years,33,34 and while the exact etiology of CSC is still not completely understood, it is believed to involve thickening of the choroid, choroidal vasculopathy and RPE defects that result in the characteristic leakage of fluid through the RPE.33 Further, CNV can be secondary to CSC, with a similar presentation to neovascular AMD associated CNV, and potentially result in severe vision loss.33 As such, the proposed mechanism for treatment of chronic CSC is closure of the abnormal leaking choroidal vessels, allowing subsequent vascular remodeling of the choroid.2 While laser photocoagulation is one method for achieving this, it has been associated with significant adverse events such as symptomatic scotomas, RPE atrophy and secondary CNV.2
As an alternative to laser photocoagulation, vPDT has demonstrated efficacy not only in promoting the resolution of acute CSC (i.e., enabling reabsorption of subretinal fluid and improving VA), but also preventing recurrences and benefitting patients with chronic disease.33 There are a large number of published studies on the use of vPDT to treat both acute and chronic CSC, and while most of these are interventional case series,35 there have also been several randomized controlled trials using adapted ‘standard’ vPDT protocols to improve safety outcomes (Table 2).36–43 For the treatment of acute CSC, these include a randomized, double-masked, controlled clinical trial by Chan et al. investigating half-dose vPDT therapy (3 mg/m2, fluence 50 J/cm2) in 63 patients with subretinal fluid for <3 months; Table 2).36 In this study, significantly more patients who received vPDT therapy had no subretinal fluid visible using OCT after 1 year compared with placebo (30 mL saline solution; 94.9% versus 57.9%; p=0.001), and significantly more patients had stable or improved VA in the vPDT group compared with placebo (100.0% versus 78.9%; p=0.009).36 No ocular or systemic adverse events were reported.
For the treatment of chronic CSC, these include a randomized trial conducted by Bae et al. in 32 patients who had subretinal fluid for >6 months, which compared vPDT (fluence: 25 J/cm2; light dose 300 mW/cm2) and ranibizumab (three injections of 0.5 mg; Table 2).37 At 12 months, significantly more eyes in the vPDT group compared with the ranibizumab group maintained complete resolution of subretinal fluid with rescue medication (88.9% versus 12.5%; p<0.001).37 Adverse events were reported only in the ranibizumab group (three eyes; all events secondary to intravitreal injections) and there were no serious adverse events.37 For patients with chronic CSC recalcitrant to conventional therapy (including anti-VEGF monotherapy), a retrospective analysis by Asahi et al. showed that combination therapy with vPDT + anti-VEGF therapy led to complete resolution of subretinal fluid in all patients (100%), with a significant reduction in mean central macular thickness after 4 months (401 μm to 298 μm; p=0.001).38 In these cases, it was thought that the efficacy observed may be due, in part, to vPDT-sensitive lesions associated with underlying CNV, something which has been reported in cases of chronic CSC.38 In addition, combining anti-VEGF vPDT can have an additive effect on the resolution of chronic CSC by further stimulation of fluid absorption, and may serve as prophylaxis against iatrogenic CNV, as well as treatment of any underlying occult CNV. Overall, the authors concluded that associated CNV and/or inflammation may be the reason for greater success in CSC patients treated with combination therapy.38
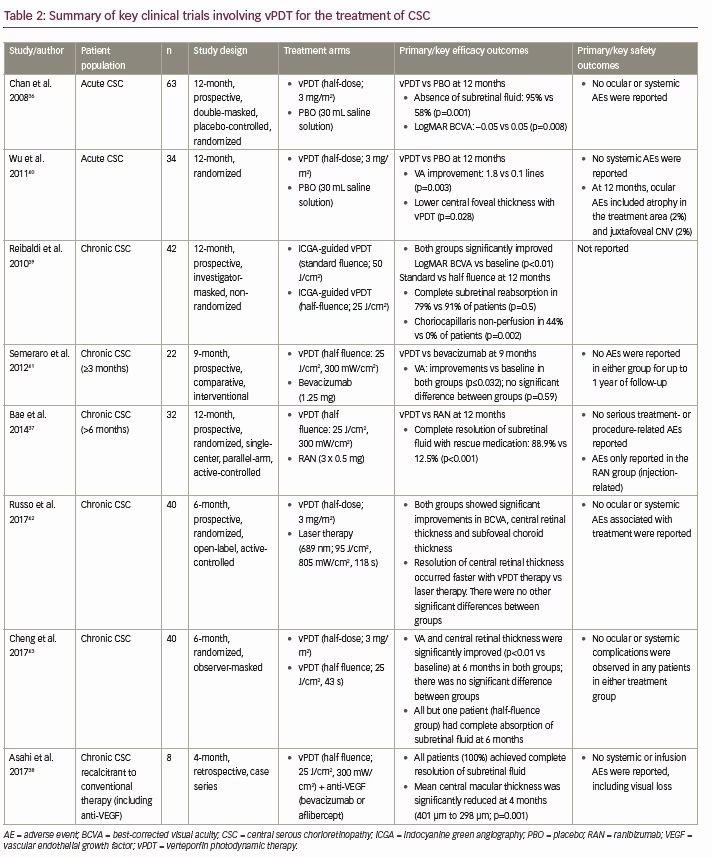
In addition, Ma et al. conducted a meta-analysis of nine randomized controlled trials (319 eyes) and observational studies comparing vPDT with both laser photocoagulation therapy and anti-VEGF therapy.44 Results of this meta-analysis showed that half dose vPDT (3 mg/m2) significantly improved the resolution of subretinal fluid versus laser photocoagulation (p=0.005), and that vPDT significantly improved the resolution of subretinal fluid and decreased central macular thickness versus anti-VEGF therapy (p=0.007 and p=0.002, respectively).44 A further meta-analysis of three studies found that eyes of patients who received vPDT showed better BCVA and central macular thickness than those who received placebo for 12 months.45 A meta-analysis of a further two studies showed that BCVA at the first month after injection was better for anti-VEGF than for placebo but not at 3 and 6 months after injection.45 This analysis found no direct comparisons of vPDT with anti-VEGF for acute CSC. No severe complications were reported in the included studies. The analysis concluded that current evidence suggested that early treatment of acute CSC by vPDT is valuable in improving VA, reducing subretinal fluid, and maintaining long term effectiveness. It was also concluded that anti-VEGF injection could shorten the duration of symptoms and accelerate visual improvement at early stage of disease.45 This analysis and another review concluded that a direct comparison between these two treatments is needed to fully evaluate their relative efficacy.45,46 While not currently indicated for the treatment of CSC, vPDT has been extensively studied in this condition, is considered the current standard of care alongside focal laser therapy,33 and was granted orphan drug designation for the potential treatment of chronic and recurrent CSC by the US Food and Drug Administration (FDA) in 2012.47
Polypoidal choroidal vasculopathy
PCV is an exudative maculopathy characterized by an inner choroidal branching vascular network with polypoidal lesions.2 Typically, the condition manifests as recurrent episodes of serous and/or hemorrhagic RPE and retinal detachment, and if left untreated, the long-term visual prognosis is poor.2,48 While it is established that PCV is prevalent in Asian individuals, occurring in 22.3–61.6% of patients with neovascular AMD,48 it occurs across all racial/ethnic groups and there is evidence that it may be more prevalent in other ethnic populations than previously thought, with the increased use of indocyanine green angiography and other diagnostic techniques showing a rise in the frequency of PCV diagnoses across all patient populations.48 Although the etiology of PCV is not fully understood, it is believed to be due to atherosclerosis of the choroidal vessels.2 As such, the optimal approach to treatment of PCV remains uncertain, though histopathological evidence suggests that PCV may be a variant of the CNV seen in neovascular AMD, indicating that a similar therapeutic approach may be viable.48 Indeed, PCV can often be misdiagnosed as CNV, as it can mimic CNV on fundus photography and fluorescence fundus angiography.49 Because the role of VEGF in the pathogenesis of PCV is believed to be substantially less important than in CNV,49 it is possible that many cases of anti-VEGF-treatment-resistant AMD may have a driving PCV pathology, and alternative treatment methods, such as vPDT, may be indicated.
Several randomized clinical trials have demonstrated the efficacy of vPDT (with or without anti-VEGF therapy) for improving visual outcomes and achieving polyp regression in patients with PCV (Table 3).48,50–54 These include EVEREST, a 6-month, randomized study investigating the use of vPDT (standard) + ranibizumab (3 x 0.5 mg) combination therapy versus vPDT or ranibizumab monotherapy in Asian patients with symptomatic PCV,50 and the 24-month, similarly-designed, follow-up study: EVEREST II. The EVEREST study (n=61) showed that both vPDT + ranibizumab and vPDT monotherapy were superior to ranibizumab monotherapy in achieving complete polyp regression at 6 months (77.8% and 71.4% versus 28.6%; p<0.01; Table 3). This improved efficacy was not associated with any new safety findings. More recently, 1-year results from the 2-year, multicenter, randomized, double-masked, EVEREST II study (n=322) have been made available. These showed that in Asian patients with PCV, vPDT + ranibizumab (3 x 0.5 mg) provided significant improvements in VA versus ranibizumab alone (mean difference at 1 year: 3.2 letters, 95% confidence interval [CI]: 0.4–6.1), meeting both non-inferiority and superiority criteria (p=0.01; Table 3).48 In addition, combination therapy was also superior to ranibizumab monotherapy in achieving complete polyp regression (69.3% versus 34.7% at 1 year; p<0.001). The incidence of ocular adverse events was similar between treatment groups (26.7% versus 25.5%), with vitreous hemorrhage the only ocular serious adverse event reported (vPDT + ranibizumab: 1 [0.6%]; ranibizumab monotherapy: 3 [2.0%]).48
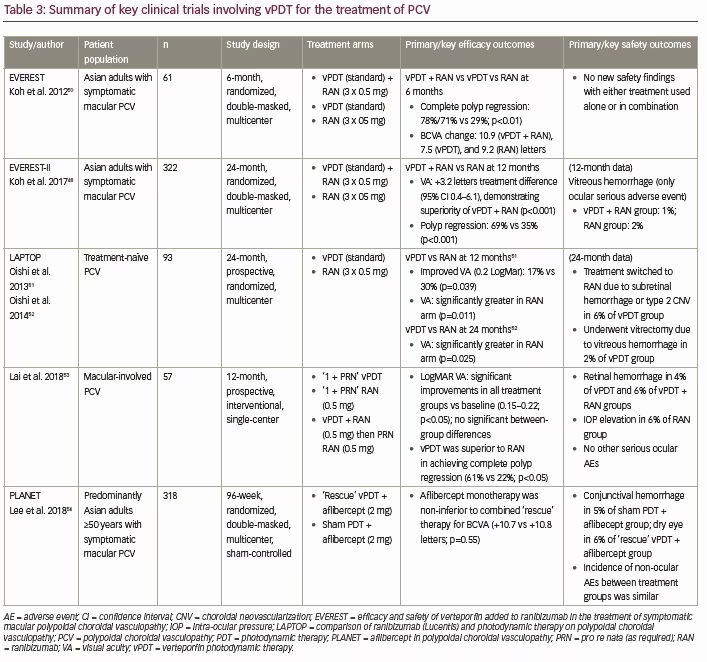
In addition to randomized clinical trials, Wong et al. conducted a systematic review of the literature for studies investigating the use of vPDT both with and without anti-VEGF therapy for the treatment of PCV.55 Overall, a pooled analysis of 29 studies (316 eyes reporting 3-year visual outcomes) showed significant improvements in VA at both 1 and 2 years post-treatment with vPDT + ranibizumab versus vPDT monotherapy.55 These findings at 1 and 2 years post-treatment were supported by a separate systematic literature review and meta-analysis conducted by Wang et al., who showed that combination therapy with vPDT + anti-VEGF provided significantly improved VA at 1 and 2 years compared with vPDT monotherapy in patients with PCV (p=0.028 and p=0.008, respectively).56
Similar to the situation with CSC, while vPDT is not currently indicated for the treatment of PCV, the numerous studies supporting the effectiveness of treatments, taken together with the results of the recent EVEREST-II study, suggest that combination therapy with vPDT + anti-VEGF may be the optimal treatment for patients with PCV, reflected in their inclusion in evidence-based treatment guidelines.57
The benefit of vPDT rescue therapy in PCV was also evaluated in the PLANET study (Table 3).54 A total of 318 patients with PCV (mean age 70.6 years) were randomized 1:1 to receive 2 mg of aflibercept at weeks 0, 4, and 8. At week 12, patients with a suboptimal response were randomized 1:1 to receive aflibercept + sham vPDT or a “rescue” of aflibercept plus vPDT. The results showed that aflibercept monotherapy was noninferior to aflibercept + vPDT for BCVA (+10.7 versus +10.8 letters, respectively; 95% CI, –2.9 to 1.6; p=0.55). However, fewer than 17% of patients met the criteria of a suboptimal response to receive rescue vPDT so the potential benefit of adding PDT could not be determined.54,58
Circumscribed choroidal hemangioma
Choroidal hemangiomas are benign vascular hematomas that are usually circumscribed, but can also be diffuse (e.g., with Sturge-Webber syndrome),59 and typically only require treatment when vision is affected by macular edema or exudative retinal detachment.2 Various treatments have been previously used to treat choroidal hemangiomas (e.g., laser photocoagulation, radiation therapy).2,59 However, as vPDT was initially developed to treat tumors via selective destruction of the tumor or tumor vasculature, it provides a clear alternative therapeutic option.2
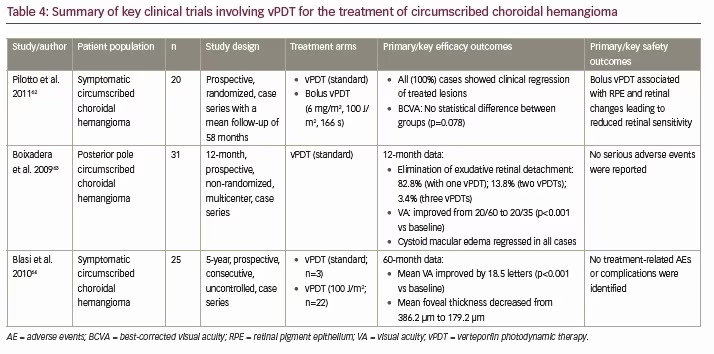
There have been many case reports and case series demonstrating the use of vPDT to effectively treat choroidal hemangiomas with minimal complications,2,60,61 but only one controlled, comparative case series: a prospective, randomized, interventional study by Pilotto et al. (Table 4).62 In this study, 20 consecutive cases of choroidal hemangioma were randomized to receive vPDT (standard) or bolus vPDT (a 6 mg/m2 verteporfin bolus over 1 minute, laser treatment after 5 minutes using 100 J/m2 over 166 s).62 All cases (100%) showed clinical regression in treated lesions and there was no difference in BCVA between groups (p=0.078).62 However, bolus vPDT was associated with RPE and retinal changes that were associated with reduced retinal sensitivity.62
Of the uncontrolled case series, the largest of these was a prospective, uncontrolled interventional case series investigating the use of vPDT (standard) in 31 patients with circumscribed choroidal hemangiomas conducted by Boixadera et al. (Table 4).63 After 12 months, cystoid macular edema had regressed in all cases and exudative macular detachment resolved in all but two cases, with most (82.8%) patients requiring only one vPDT treatment to eliminate exudative retinal detachment.63 Mean VA increased from 20/60 at baseline to 20/35 at 12 months (p<0.001), with 69% of patients demonstrating visual recovery (p<0.001 versus baseline).63 Choroidal hemangioma thickness decreased in all cases from a mean of 3.0 mm to 1.7 mm at 12 months, with the greatest effect seen after 4 weeks of treatment (p<0.001 versus baseline).63 No severe adverse events were reported.
With regard to modified vPDT protocols, Blasi et al. conducted a prospective, consecutive, uncontrolled, interventional case series investigating the use of vPDT (standard in 3/25 patients; double [100 J/cm2] light dose in 22/25 patients) for the treatment of circumscribed choroidal hemangiomas (Table 4).64 The three eyes treated with standard vPDT received a second vPDT session with a 100 J/cm2 light dose 1 month after the initial session. After 60 months’ follow-up, mean VA had improved by 18.5 letters (Early Treatment Diabetic Retinopathy Study VA test; p<0.001 versus baseline), mean foveal thickness had decreased from 386.20 μm to 179.2 μm, macular exudation was completely resolved in all cases, and all tumors were reduced in size.64 Similar to the findings of Boixadera et al., no treatment-related adverse events or complications were identified.64 In addition, although not a true choroidal disease, disc and retinal angiomatosis is also a clinical condition that has been reported to be successfully treated with vPDT.65,66
Overall, clinical evidence indicates that vPDT (both standard and double light doses) is an effective treatment for circumscribed choroidal hemangiomas, though radiation therapy may still be required for larger or more diffuse tumors with extensive exudative retinal detachment that are not suitable for vPDT therapy.2,59
Peripapillary choroidal neovascularization
Peripapillary CNV is usually idiopathic and is defined as CNV located within one disc diameter of the optic nerve head.2 Conventional treatment with laser photocoagulation has been associated with an increased risk of thermal injury to the overlying neurosensory retina and visual loss.2 As such, vPDT could provide an effective alternative with a more favorable safety profile.
In line with this hypothesis, case series have shown that one or two treatments with vPDT can lead to regression of peripapillary CNV with only a small risk of adverse events (Table 1),67,68 whereas anti-VEGF therapy may require multiple intravitreal injections to achieve regression.69,70 In addition, while it has been advocated that the laser spot should not extend to within 200 μm of the optic nerve in the treatment of CNV-associated AMD,2 there is some evidence that the optic head may be included in the vPDT treatment zone without causing optic nerve damage.68 Overall, vPDT provides an effective alternative to conventional photocoagulation therapy in peripapillary CNV with an improved safety profile, though further studies are warranted to confirm the findings and characterize the optimal vPDT protocol.
Conclusion
vPDT is a selective vaso-occlusive treatment that targets choroidal vascular abnormalities, which was initially used in ophthalmology to treat CNV associated with AMD.2 While anti-VEGF therapy has now replaced vPDT as the modality of choice for the treatment of AMD-associated CNV, clinical evidence indicates it is still an important treatment option for a range of choroidal conditions (either with or without anti-VEGF therapy), such as CSC, PCV, choroidal hemangioma and peripapillary CNV. In particular, vPDT used as an adjunct to anti-VEGF therapy can help to reduce the intravitreal injection burden and provide efficacy in patients with conditions refractive to anti-VEGF monotherapy, and numerous studies suggest that vPDT + anti-VEGF therapy may be the optimal treatment for patients with PCV. As such, further research is required to optimize the vPDT protocols for these conditions, varying aspects such as verteporfin dose and laser fluency to obtain the best balance between treatment efficacy and safety.







