Diabetic retinopathy (DR) is the main cause of vision loss in people between 25 and 74 years old in developed countries, and diabetic macular oedema (DMO) represents the major cause of this visual impairment.1,2
The prevalence of DMO ranges from 0 % to 3 % at the moment of the diagnosis of diabetes to 28 % to 29 % in patients with diabetes duration of over 20 years.3
DMO is the result of blood–retinal barrier disruption and extravascular inflammation and may arise in the setting of both non-proliferative and proliferative DR. DMO has been recently classified in four main forms: vasogenic DMO, as retinal thickening with vascular dilations and hard exudates; non-vasogenic, defined by retinal thickening without identifiable vascular dilations; tractional DMO, due to vitreomacular abnormal adhesion; and mixed DMO, when tractional DMO is associated with vasogenic or non-vasogenic forms.4
Several studies, including the Diabetes Control and Complications Trial (DCCT) for type 1 and the United Kingdom Prospective Diabetes Study (UKPDS) for patients with type 2 diabetes, have shown that primary prevention, including strict blood glucose, blood pressure and serum lipids control, reduces the incidence and severity of DR and DMO.5
Laser photocoagulation is still considered the standard of care for DMO since 1985, and can reduce the risk of moderate visual acuity (VA) loss by about 50 %, although only 3 % of eyes achieve a vision improvement (≥3 lines), and a substantial proportion of treated eyes remain unresponsive.6 Vitreo-retinal surgery, and in particular pars plana vitrectomy and epiretinal membrane peeling, has generally limited efficacy for tractional forms of DMO, being responsible for remarkable side effects.7
Recent advances in understanding DMO pathophysiology have identified the primary role of vascular endothelial growth factor (VEGF) in retinal vasopermeability regulation and extracellular fluid accumulation.8 Hyperglycaemia-related ischaemic changes and lowgrade inflammation at a retinal level induce VEGF genes up-regulation and protein translation; in fact, high levels of VEGF have been found in vitreous samples from the eyes of patients with diabetes.9
VEGF is a pluripotent cell mitogen that causes neo-angiogenesis and disruption of the intercellular tight junctions normally present between retinal endothelial cells, leading to intraretinal leakage.10 Its family includes five molecules: placental growth factor, VEGF-A, VEGF-B, VEGF-C and VEGF-D. VEGF-A, especially the VEGF165 isoform, plays a primary role in the pathogenesis of DMO. For this reason it represents an interesting candidate as a therapeutic target for the treatment of DMO, being the VEGF receptor activation blockage the most targeted therapeutic strategy.
Recent multicentre randomised clinical trials (RCTs) have demonstrated that intravitreal injections of steroids or anti-VEGF molecules result in a significant gain of VA in eyes with DMO, and preserve patients with diabetes from progression towards more advanced forms of DR.11–12 Pegaptanib, ranibizumab, bevacizumab and aflibercept are the most investigated VEGF-inhibitors in recent published literature.13 Pegaptanib is a pegylated 28-nucleotide RNA aptamer that binds to VEGF165 with high specificity; ranibizumab and bevacizumab are a 48 KDa antigen-binding fragment (Fab) and a humanised anti-VEGF antibody, respectively, that inhibit all biologically active isoforms and active proteolytic fragments of VEGF-A. Aflibercept is a 115 kDa fusion protein of portions of VEGF receptor 1 and 2 fused to the constant region of human immunoglobulin (Ig)-G1, functioning as a soluble decoy receptor or all VEGF-A isoforms, with a higher affinity (140 times stronger) and longer half-life in comparison to all the others anti-VEGF substances.
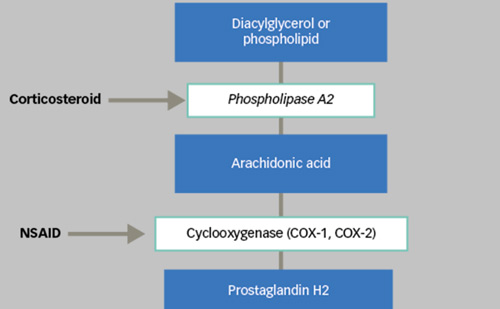
Corticosteroids reduce the breakdown of the blood–retinal barrier, have anti-inflammatory and anti-angiogenic properties and experimentally have been disclosed to down-regulate VEGF production; triamcinolone acetonide, fluocinolone and dexamethasone are the three most used steroidal molecules in DMO management.14
The aim of this article is to review the role of ranibizumab in the management of DMO, focusing on the most important trials that have proved its efficacy and safety.
Ranibizumab
Ranibizumab (Lucentis; Genentech USA Inc., San Francisco, CA, US/ Novartis Ophthalmics, Basel, Switzerland) is a Fab derived from bevacizumab, but it is smaller and specifically designed for use in the eye.
In 2012, intravitreal ranibizumab (IVR) was US Food and Drug Administration (FDA)-approved to treat DMO, since its beneficial effects have been shown by two phase II studies (RESOLVE and READ-2) and five phase III studies (RISE, RIDE, RESTORE DRCR.net protocol I and REVEAL) compared efficacy of ranibizumab with sham (RISE, RIDE and RESOLVE) or with laser photocoagulation (READ-2, RESTORE, DRCR.net protocol I and REVEAL).
The RISE (n=377) and RIDE (n=382) trials followed identical protocols, comparing monthly injections of 0.3 mg IVR or 0.5 mg IVR with sham injection for 24 months. At 24 months, in the RISE group, 44.8 % of 0.3 mg patients and 39.2 % of 0.5 mg patients gained ≥15 letters, compared with 18.1 % of sham, while in RIDE, 33.6 % of 0.3 mg patients and 45.7 % of 0.5 mg IVR patients gained ≥15 letters compared with 12.3 % of sham.15 In the third year, the study design allowed for patients in the sham group to cross-over and receive monthly IVR; however, delayed treatment in these patients resulted in the lower functional recovery in terms of patients originally randomised to IVR.16
The RESOLVE study, a 12-month randomised, double-masked, multicentre, phase II study, investigated the most effective concentration of ranibizumab through comparing 151 eyes with DMO randomly assigned to two different groups of IVR (0.3 or 0.5 mg; n=51 each) or sham (n=49). The treatment schedule comprised three consecutive monthly injections, followed by repeated treatment or rescue laser photocoagulation in case of refractory oedema. At 1-year follow-up, mean best-corrected VA (BCVA) improved from baseline by 11.8 letters and 8.8 letters in the 0.3 or 0.5 mg group, respectively; pooled data (including both dosing regimens) showed a total gain of 10.3 letters from baseline. BCVA declined by 1.4 letters with sham and only 4.9 % of IVR patients required rescue laser compared with 34.7 % of sham.17
Ranibizumab plus Laser Combination Treatment
Combination treatment has been recently analysed in a meta-analysis pooling data from seven RCTs (see Table 1) and 1,749 patients (394 patients in the IVR group, 642 in the IVR + laser group and 713 in the laser group).18 One trial (the LUCIDATE study) was a single-centre trial, while the others were all multicentre trials. IVR and IVR + laser were superior to laser monotherapy in the mean change of BCVA and central retinal thickness (CRT) from baseline. The pooled relative risk (RR) comparing the proportions of patients with at least 15 letters improvement were
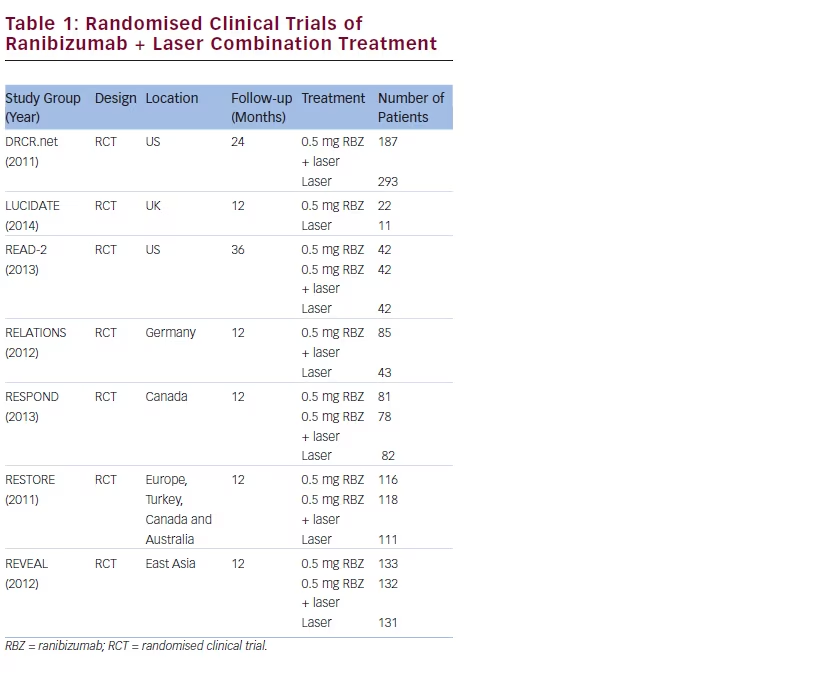
also in favour of IVR and IVR + laser (RR = 2.94; p<0.00001 and RR = 2.04; p<0.00001); same results for at least 15 letters deterioration (RR = 0.21; p=0.01 and RR = 0.52; p=0.03, respectively). There were no significant differences between IVR and IVR + laser for any of the parameters. This analysis showed that the mean number of ranibizumab tended to be less in IVR + laser arm in terms of IVR monotherapy (6.8 versus 7.0 injections in the RESTORE study at 1 year and 3.3 versus 5.4 injections in the READ-2 study at 3 years).
In detail, the READ-2 was a phase II, prospective, randomised clinical trial conducted at 14 sites in the US, designed to compare IVR monotherapy with combination therapy with focal/grid laser in patients with DMO. One hundred and twenty-six patients were randomised to receive 0.5 mg IVR (n=42), focal/grid laser photocoagulation (n=42) or a combination of 0.5 mg of ranibizumab and focal/grid laser (n=42).19 Ranibizumab was administered at baseline and at months 1, 3, 5, and focal/grid laser was administered at baseline and again at month 3 only if central subfoveal thickness (CST) was ≥250 μm. The change in BCVA at month 6 compared with baseline in the IVR monotherapy group was higher (+7.24 letters) than the laser monotherapy (–0.43 letters) and combination (+3.8 letters) groups. At month 24, the 101 patients remained in the study showed a mean BCVA improvement of 7.7 letters in the IVR-only group (n=33), 5.1 letters in the laser (n=34) and 6.8 letters in the combination group (n=34). The combination therapy arm resulted in a smaller need of injections to maintain visual outcome at year 2 and 3.20
RESTORE, a 12-month, double-masked, multicentre, laser-controlled, phase III study, demonstrated the superiority in 2-year follow-up of IVR 0.5 mg, both as monotherapy and combined with prompt or deferred
Early Treatment Diabetic Retinopathy Study Group (ETDRS) laser treatment compared with laser treatment alone.21 Three hundred and forty-five patients with DMO were randomised to IVR monotherapy (n=116), laser monotherapy (n=111) or ranibizumab combined with laser (n=118). Ranibizumab was administered monthly for 3 months and then on a pro re nata (PRN) basis. At month 12, CRT was significantly reduced compared with baseline with IVR alone (–118.7 μm) or combined with laser (–128.3 μm) versus laser only (–61.3 μm). A significantly greater proportion of patients had a BCVA letter gain ≥15 and BCVA letter score level >73 (20/40 Snellen equivalent) with IVR (22.6 % and 53 %, respectively) and IVR + laser (22.9 % and 44.9 %) versus laser only (8.2 % and 23.6 %). Moreover, there was a significantly greater BCVA improvement in the ranibizumab monotherapy (+6.1 letters) and the combined group (+5.9 letters) compared with the laser arm (+0.8 letters). The RESTORE 2-22 and 3-year extension23 study found that the BCVA gained in the prior laser group was less than that observed in the prior IVR groups after allowing IVR in all groups (5.4 letters in prior laser group, 6.7 letters in prior IVR + laser group and 7.9 letters in prior IVR group at month 24 and 6.0 letters in prior laser group, 6.7 letters in prior IVR + laser group and 8.0 letters prior IVR group at month 36). At month 36, the patients in the prior laser group demonstrated a BCVA gain similar to that observed in the prior ranibizumab groups, but the BCVA gain observed in the prior laser group over a period of 3 years turned out to be more gradual compared with the rapid initial gain observed in the prior ranibizumabtreated patients. Patients who received ranibizumab previously in the core study and continued with ranibizumab treatment in the extension study (prior ranibizumab and prior ranibizumab + laser) received a median of 12.0 ranibizumab injections over 3 years. However, the mean number of ranibizumab injections administered over 2 years of the extension study (months 12–35) was similar across the three groups, with a progressive reduction in retreatment need from the first to the second year and the third year (median, 6.0; mean, 6.8 in prior ranibizumab; median, 4.0; mean, 6.0 in prior ranibizumab + laser, and median, 4.0; mean, 6.5 in prior laser group).
The REVEAL study, a 12-month, double-masked, multicentre, lasercontrolled, phase III study followed the same design, randomising 396 Asian patients to ranibizumab + sham laser (n=133), ranibizumab + active laser (n=132) or sham injection + active laser (n=131). Ranibizumab/ sham injections were administered on day 1 and continued monthly.24 As of month 3, monthly injections were continued if stable vision was not reached. Treatment was reinitiated if BCVA decreased because of DMO progression. Active/sham laser was administered on day 1 and thereafter according to ETDRS guidelines. Ranibizumab monotherapy or combined with laser was superior to laser in improving BCVA from baseline to month 12 (+5.9 and +5.7 versus +1.4 letters, respectively). At month 12, a greater proportion of patients gained ≥15 letters with ranibizumab and ranibizumab + laser compared with laser (18.8 % and 17.8 % versus 7.8 %). Mean CRT reduced significantly from baseline to month 12 with ranibizumab (–134.6 μm) and ranibizumab + laser (–171.8 μm) versus laser (–57.2 μm). Patients received a mean of 7.8 and 7.0 ranibizumab injections in the ranibizumab and ranibizumab + laser arms, respectively, and 1.5–1.9 active laser across treatment arms.
Finally, combination treatment of steroidal or ranibizumab with prompt or deferred (≥24 weeks) focal/grid laser was investigated by the Diabetic Retinopathy Clinical Research Network (DRCR.net).25 A phase III multicentre clinical trial randomised 691 patients (854 eyes) with DMO in four groups: laser monotherapy (293 eyes), 0.5 mg IVR + prompt laser photocoagulation (187 eyes), 0.5 mg IVR + deferred laser (at least 24 weeks, 188 eyes), intravitreal triamcinolone 4 mg + prompt laser (186 eyes). IVR was administered monthly for 3 months, and then PRN on individual bases. Intravitreal ranibizumab with prompt or deferred laser and triamcinolone with prompt laser provided superior VA outcomes (respectively +9 letters, +9 letters and +4 letters) compared with laser monotherapy (+3 letters) for at least 2 years; a median of two and three injections in the ranibizumab plus prompt and deferred laser groups, respectively, was reported. There was a greater risk of 10- and 15-letter vision loss in the laser-only cohort (13 % and 8 %, respectively) versus the ranibizumab cohorts (4 % and 2 %, respectively). Elevated intraocular pressure and cataract surgery were more frequently observed in the triamcinolone group in comparison to groups receiving ranibizumab + laser or laser alone.
Regimen of Treatment
As far as it regards the correct regimen of treatment, two recent phase IIIb multicentre studies (RETAIN and RELIGHT) have respectively investigated ranibizumab schedule on the bases of a treat-and-extend (TE) regimen and a bimonthly follow-up with PRN regimen.
RETAIN, a 24-month single-masked study, explored the non-inferiority/ superiority of a TE regimen, with/without laser, to a PRN regimen in patients with visual impairment due to DMO.26 Three hundred and seventy-two eyes were randomised to TE ranibizumab + laser (n=121), TE ranibizumab (n=128) or PRN ranibizumab (n=123). In phase A, all patients received monthly ranibizumab until VA stability. In phase B, the therapy-free interval was incrementally extended by 1 month (up to 3) in VA-stable patients in TE groups. PRN patients were monthly visited in phase B. Both TE groups were non-inferior to PRN based on mean BCVA change from baseline to month 12 (+5.9 and +6.1 versus +6.2 letters; both p<0.0001) and to month 24 (+8.3, +6.5 and +8.1 letters, respectively). The median injection number over 24 months was 12 for TE and 10 for PRN. There was approximately a 40 % reduction in visits scheduled with TE, since approximately 70 % of TE patients had a monitoring interval of ≥2 months.
RELIGHT, an 18-month, prospective, open-label, single-arm, study used the impact of bimonthly follow-up and individualised retreatment, after a monthly follow-up for 6 months on maintaining improvements in BCVA.27 Patients received three initial monthly IVR injections, followed by individualised VA- and optical coherence tomography (OCT)-guided retreatment with monthly (until month 5), and subsequent bimonthly follow-up (months 6 to 18). The study demonstrated that VA gains were maintained with a bimonthly monitoring regimen over 12 months, allowing a lower number of monitoring visits as a viable option for the long-term management of DMO. In fact, mean change in BCVA was +4.9 letters (month 12) and +6.5 letters (month 18) achieved with a mean of 6.8 and 8.5 injections, respectively. CRT decreased from –127 mm (month 12) to -150 mm (month 18). The proportion of patients gaining ≥10 and ≥15 letters were 24.8 % and 13.8 % at month 12 and 35 % and 19 % at month 18, respectively. A subgroup analysis on BCVA based on the duration of DMO shows that patients with shorter duration of DMO (<12 months) achieved greater BCVA improvements compared with patients with a longer duration of DMO (>12 months).
Safety
Globally, these trials have showed that patients randomised to ranibizumab were more likely to experience improvements in DR severity as measured by ETDRS retinopathy severity scale and less likely to develop proliferative DR. However, IVR injections have shorter duration of action and higher recurrence rate of the disease compared with laser treatment, therefore requiring more frequent treatments; higher frequency of treatments automatically exposes patient to a higher rate of side effects, connected to both surgical procedure and systemic exposure to the drug. The most common side effects connected to the surgical procedure are conjunctival haemorrhage, eye pain, vitreous floaters, increased intraocular pressure and intraocular inflammation; more severe complications, including endophthalmitis, retinal tears and holes and vitreous haemorrhage, occurred with an incidence rate of less than 1 %.28 Patients with DMO and co-existing proliferative DR have showed an increased risk of vitreal fibrosis and consequent tractional retinal detachment.
Although anti-VEGF therapy has shown a favourable tolerability profile in patients with diabetes, research has shown that a little amount of intravitreal anti-VEGF drugs pass into systemic circulation, and may increase the risk of non-ocular adverse events, including thromboembolic events (TEE) and hypertension (HTN). Different RCTs have tried to evaluate anti-VEGF molecules in terms of relative efficacy and systemic safety.29,30 Recently, the 2-year Comparison of the AMD Treatments Trial (CATT)31 and the Age-related Choroidal Neovascularization Trial (IVAN)32 have performed a head-to-head comparison between ranibizumab and bevacizumab in treatment of age-related macular degeneration. In IVAN, there were more atherothrombotic events and heart failure with ranibizumab. In CATT, adverse events (including infections, palpitations and accidents) were more frequent with bevacizumab than ranibizumab (24 % versus 19 % at 1 year, and 40 % versus 32 % at 2 years).
Ranibizumab is, nowadays, generally avoided in patients with a history of stroke or myocardial infarction, even if recent meta-analyses of clinical trials have shown no increased risk of ranibizumab and no significant differences between bevacizumab and ranibizumab in terms of incidence of death from all causes, atherothrombotic events, stroke, non-fatal myocardial infarction, vascular death, venous thrombotic events and hypertension.
Economic Burden
Concerns on ranibizumab injections have beed raised about its economic burden compared with other anti-VEGF agents. A comparison between the costs of these agents has shown that $1,950 per dose for ranibizumab, $1,850 per dose for VEGF-trap eye, $995 per dose for pegaptanib and less than $50 per dose for bevacizumab are needed. The direct ophthalmic medical cost for ranibizumab therapy in the US has been estimated $30,116 for first eye, $26,220 for second eye and $56,336 for bilateral therapy.33 (At time of publication $1 = €0.90.)
The costs of bevacizumab are 20 to 40-fold lower than those of ranibizumab, and it has been estimated that in each European country, the costs of ranibizumab treatment of DMO would be around 10–15 million euros higher than treatment with bevacizumab.34 Implementing a first-line therapy with bevacizumab for patients with DMO could reduce costs enormously; however, conclusive evidence from RCTs directly comparing effectiveness ranibizumab with similar molecules is lacking.
Nepomuceno et al. recently completed a head-to-head comparison in 63 eyes. Patients were treated monthly with either bevacizumab or ranibizumab for 1 year.35 They observed a significant BCVA improvement in both groups at all study visits, but this improvement was significantly greater in the ranibizumab group at week 8 and 32. There was no significant difference in decrease in CRT. The mean number of injections was significantly higher in the bevacizumab group (9.84) than in the ranibizumab group (7.67).
Trial NCT01610557, a double blind comparison of ranibizumab as monotherapy and ranibizumab and bevacizumab consecutively, is ongoing.
Ranibizumab versus Bevacizumab
versus Aflibercept
Recently, a multicentre, randomised clinical trial (PROTOCOL T), sponsored by DRCR.net, has been published comparing the relative efficacy and safety of ranibizumab in the treatment of DMO with aflibercept and bevacizumab.36 Six hundred and sixty adults with DMO were randomly assigned to receive 2.0 mg aflibercept (n=224), 1.25 mg bevacizumab (n=218) or 0.3 mg ranibizumab (n=218). The study drugs were administered every 4 weeks, according to a protocol-specified algorithm. Laser photocoagulation therapy (focal, grid or both) was initiated at or after the 24-week visit for persistent diabetic macular oedema, defined on the basis of protocol-specified criteria.
The primary outcome was the mean change in BCVA at 1 year: it turned out that all ranibizumab, aflibercept and bevacizumab improved VA in eyes with centre-involving DMO, but the improvement was greater with aflibercept than with the other two drugs (+13.3 with aflibercept, +9.7 with bevacizumab and +11.2 letters with ranibizumab from baseline; p<0.001 for aflibercept versus bevacizumab and p=0.03 for aflibercept versus ranibizumab). However, this improvement was not clinically meaningful because the difference was driven by the eyes with worse VA at baseline, and the relative effect depended on baseline VA.
In detail, when the initial BCVA was comprised in the 78 to 69 range (51 % of participants), the mean improvement was +8.0 with aflibercept, +7.5 with bevacizumab, and +8.3 with ranibizumab (p>0.50 for each pairwise comparison). When the initial BCVA was less than 69, the mean improvement was 18.9 with aflibercept, 11.8 with bevacizumab and 14.2 with ranibizumab (p<0.001 for aflibercept versus bevacizumab, p=0.003 for aflibercept versus ranibizumab and p=0.21 for ranibizumab versus bevacizumab).
The median number of injections using the protocol-specified retreatment regimen was one fewer in patients treated with aflibercept compared with bevacizumab and ranibizumab: it was nine in the aflibercept, 10 in the bevacizumab and 10 in the ranibizumab group (p=0.045 for overall comparison).
Patients in the aflibercept group received fewer criteria-based macular laser treatments than those treated with bevacizumab and ranibizumab. There were no significant differences among the study groups in the rates of serious adverse events (p=0.40), hospitalisation (p=0.51), death (p=0.72) or major cardiovascular events (p=0.56).
Conclusion
Intravitreal administration of anti-VEGF substances has revolutionised the approach of clinicians to DMO and its visual outcomes. Pegaptanib, bevacizumab, ranibizumab and, more recently, aflibercept have become first-line medical treatment for DMO characterised by central macular involvement and vision loss; however, no single treatment molecule has been proved superior to the others.
Ranibizumab has been extensively investigated recently, and has demonstrated its efficacy in improving BCVA and reducing the risk of retinopathy progression and its superiority compared with laser treatment. However, there are still concerns about its economic burden both for patients and ophthalmologists.
Moreover, there are many unanswered questions about ranibizumab, regarding doses, frequency and regimen of treatment; the potential role of laser/injection combination therapy and the association with other anti-VEGF molecules and between surgery and ranibizumab in the treatment and in the prevention of visual impairment due to DMO are also still under debate.
A limitation of this review is its focus only on ranibizumab, although other treatment options, including other anti-VEGF and steroidal molecules have been demonstrated as effective and as tolerable as ranibizumab.
In conclusion, the ranibizumab approach is a major breakthrough in the treatment of DMO; a unified accepted protocol of treatment has not been found, and an individualised patient-based approach is rather preferred. New trials are required in order to answer these clinical open questions.