Diabetic macular oedema (DMO) is responsible for much of the vision loss associated with diabetic retinopathy, the leading cause of blindness in working-age adults.1–3 DMO is characterised by thickening of the central retina due to leakage of fluid from blood vessels, and can occur at any stage of retinopathy.4 Recent estimates suggest that around 21 million people worldwide have DMO.5
Evaluation of DMO should be based on both functional (visual acuity) and anatomical criteria. Optical coherence tomography (OCT) is used to assess retinal morphology and thickness of the macula, and additional methods for anatomical assessment include biomicroscopy, fundus photography and fluorescein angiography.6,7 Although focal laser photocoagulation has been the standard of care for DMO, it can only slow progression and its ability to reverse vision loss is low;8,9 in addition, it has been associated with a risk of scarring and other thermal complications.10,11 The corticosteroids (dexamethasone, triamcinolone acetonide and fluocinolone acetonide) have also been used for treatment of persistent or refractory DMO and provide improvements in retinal thickness and visual acuity. However, steroidrelated side effects, such as elevated intraocular pressure and cataract, must be considered.12,13
Awareness of the role that vascular endothelial growth factor (VEGF) plays in the pathogenesis of retinal diseases, including DMO,14–16 has led to the development and widespread use of VEGF inhibitors in this area. Intravitreal ranibizumab (IVR) has been approved for the treatment of DMO with once-monthly dosing, whereas intravitreal aflibercept (IVT-AFL) has been approved with dose administration once every 2 months after a loading dose. IVT-AFL has recently been approved in the EU and the US for the treatment of patients with visual impairment due to DMO.17,18 Further regulatory submissions for IVT-AFL in DMO have been made in Japan, Asia-Pacific and Latin America. In addition to IVR and IVT-AFL, intravitreal bevacizumab (IVB), which is not approved for any retinal disorders, is often used off-label for the treatment of DMO. This review summarises the evidence supporting the role of IVT-AFL in the treatment of DMO.
How Does Intravitreal Aflibercept Work?
Aflibercept is composed of extracellular domains from human VEGF receptors 1 and 2 fused to the Fc portion of human immunoglobulin-G1.19 By contrast, bevacizumab is a recombinant, humanised monoclonal antibody that binds all isoforms of VEGF-A, and ranibizumab is a humanised anti-VEGF antibody fragment comprising the antigen-binding Fab moiety, without the Fc domain, which was derived from bevacizumab.20,21 These differences in design result in substantial differences in the pharmacokinetic properties of these drugs.
Aflibercept binds human VEGF-A with a markedly (approximately 100- fold) higher affinity than ranibizumab or bevacizumab: an in vitro binding study using the VEGF-A165 isoform reported a dissociation constant (KD) of 0.49 pM for aflibercept compared with 46 pM for ranibizumab and 58 pM for bevacizumab.21 In addition, VEGF receptor 1 and 2 activation and VEGF-A-induced calcium mobilisation and migration in human umbilical vein endothelial cells (HUVECs) were more potently inhibited by aflibercept than by ranibizumab or bevacizumab.21
Of particular relevance is the synergistic relationship between VEGF and placental growth factor (PlGF) in the development of angiogenesis, including the disruption of the retinal barrier during diabetic retinopathy.16,22 In vitro studies showed that aflibercept binds PlGF-2 (KD: 38.9 pM), whereas ranibizumab and bevacizumab do not. Consistent with this finding, only aflibercept inhibited PlGF-induced VEGF receptor 1 activation and HUVEC migration.21 In immortalised bovine retinal endothelial cells, proliferation induced by a combination of VEGF-A and PlGF was completely suppressed by aflibercept,23 but only partially suppressed by ranibizumab.24 All three compounds have been shown to reduce VEGF-A-stimulated migration,23,24 and therapeutically achievable concentrations of aflibercept not only normalised VEGF-A-stimulated cell migration but drove it below the level of basal migration.23
Clinical Benefits of Intravitreal Aflibercept
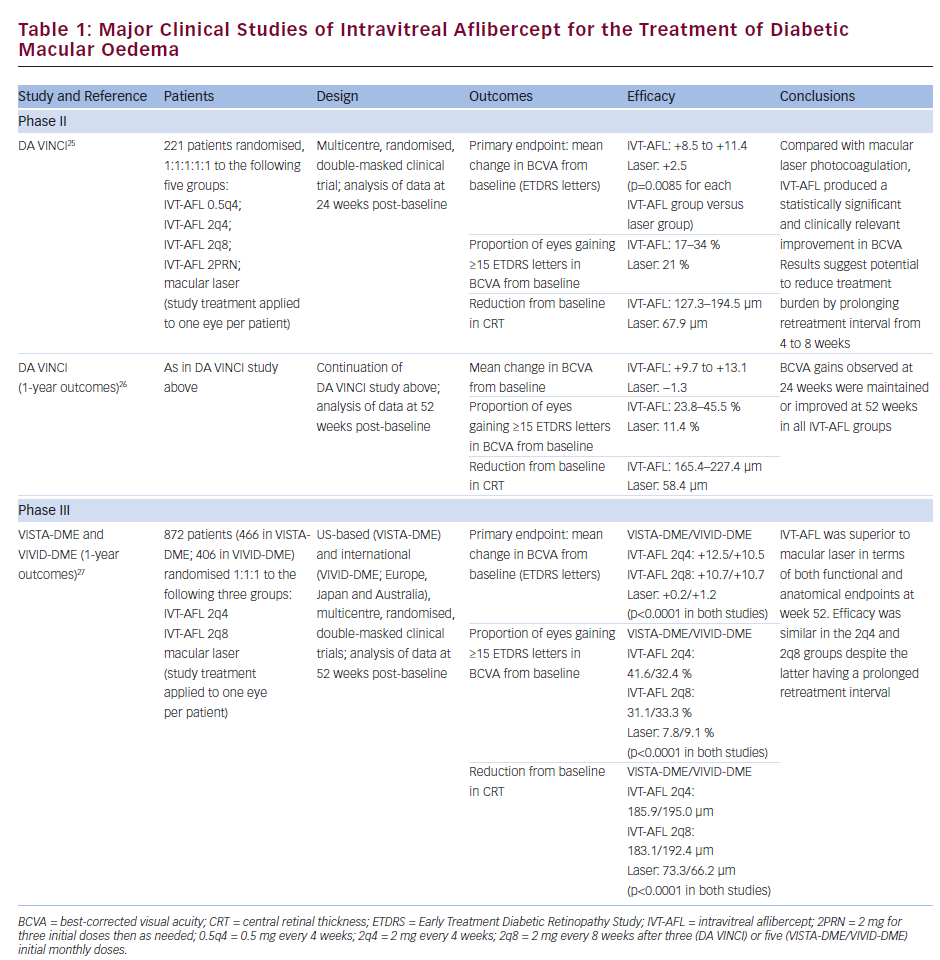
The benefits of IVT-AFL in DMO have been established in three major studies: the phase II DMO and VEGF Trap-Eye: Investigation of Clinical Impact (DA VINCI) study25,26 and the phase III studies Study of Intravitreal Administration of VEGF Trap-Eye [BAY86-5321] in Patients With Diabetic Macular Edema (VISTA-DME) and Intravitreal Aflibercept Injection in Vision Impairment Due to Diabetic Macular Edema (VIVID-DME; see Table 1).27 VISTA-DME and VIVID-DME had similar study designs but enrolled patients from different regions. All three studies enrolled adult patients with type 1 or type 2 diabetes, DMO involving the central macula and a best-corrected visual acuity (BCVA) of 73–24 letters on the Early Treatment Diabetic Retinopathy Study (ETDRS) scale (approximate Snellen equivalent: 20/40–20/320). The studies were active-controlled, comparing IVT-AFL with macular laser photocoagulation. Four IVT-AFL regimens were tested in the DA VINCI study (0.5 mg every 4 weeks; 2 mg every 4 weeks [2q4]; 2 mg every 8 weeks [2q8] after three initial monthly doses; 2 mg as needed after three initial monthly doses), and two regimens were tested in the VISTA-DME/VIVID-DME studies (2q4; 2q8 after five initial monthly doses).
Efficacy
In the DA VINCI study, patients receiving IVT-AFL experienced significantly greater ETDRS letter gains from baseline than patients receiving macular laser photocoagulation (see Table 1); these gains were observed at 24 weeks post-baseline with each IVT-AFL regimen tested,25 and were maintained or improved at 52 weeks post-baseline.26 Improvements in vision were accompanied by significant reductions in central retinal thickness (CRT; see Table 1), and at week 52, the Diabetic Retinopathy Severity Score (DRSS) had improved from baseline in 31–64 % of patients receiving IVT-AFL compared with only 12 % of patients receiving laser treatment.
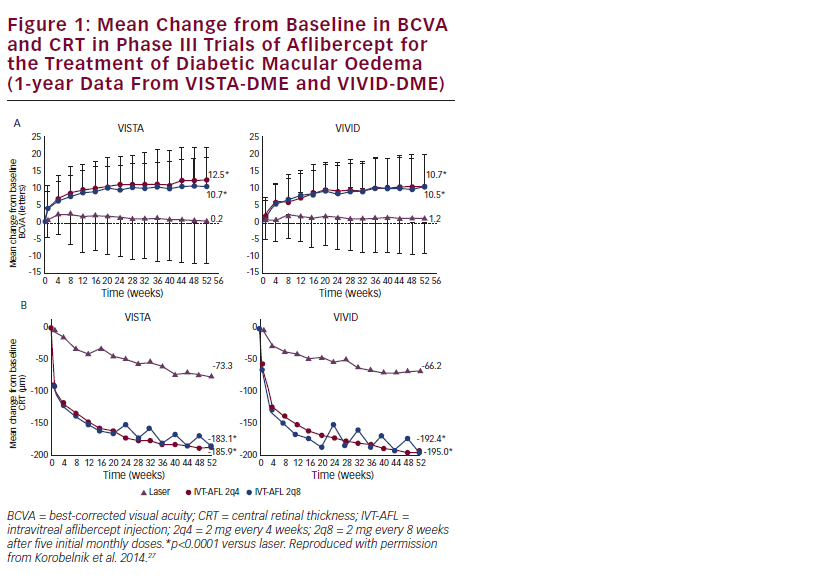
The phase III studies VISTA-DME and VIVID-DME have confirmed the promising findings of the DA VINCI study. At 52 weeks, IVT-AFL was superior to laser photocoagulation in improving BCVA and CRT from baseline (see Table 1 and Figure 1). Compared with the respective laser treatment groups, a significantly greater proportion of patients in the IVT-AFL groups experienced improvements from baseline in BCVA of 15 ETDRS letters or more in the VISTA-DME and VIVID-DME studies (see Table 1). The proportion of patients showing an improvement in the DRSS was also significantly greater in the IVT-AFL groups than in the laser groups: in VISTA-DME, an improvement of two steps or more was observed in 33.8 % and 29.1 % of the IVT-AFL 2q4 and 2q8 groups, respectively, compared with 14.3 % of the laser group (p<0.01); the corresponding proportions in VIVID-DME were 33.3 % and 27.7 % in the IVT-AFL groups compared with 7.5 % in the laser group (p<0.001).27 Although not yet fully published, data on longer-term outcomes in the VISTA-DME and VIVID-DME trials suggest that the statistically significant benefits over laser achieved at week 52 were maintained through 100 weeks.28
IVT-AFL has been indirectly compared with IVR in several meta-analyses. A Cochrane meta-analysis found no significant difference between IVTAFL and IVR in terms of the percentage of patients experiencing an improvement in BCVA of 15 or more ETDRS letters, although the authors noted that there was little power to detect such a difference.29 Another meta-analysis reported that IVR was numerically but not statistically superior to IVT-AFL in terms of the percentage of patients experiencing an improvement in BCVA of 10 or more ETDRS letters.30 Analysis of the mean change from baseline in BCVA has produced more consistent results, with two meta-analyses reporting a greater degree of improvement with IVT-AFL than with IVR.29,31 The most recent meta-analysis, which used a robust methodology to identify a network of up to 10 studies involving
3,060 patients with DMO, showed that IVT-AFL 2q8 improved the mean 12-month change in BCVA to a greater extent than IVR 0.5 mg pro re nata; there was a four- to fivefold difference between the two agents in both mixed-treatment comparisons (mean difference was 4.67 letters) and in Bucher indirect comparisons (mean difference was 4.82 letters).31
IVT-AFL has also been directly compared with IVR and IVB in a recent US National Institutes of Health-sponsored trial: the Diabetic Retinopathy Clinical Research Network (DRCR.net) comparative effectiveness study in patients with DMO (Protocol T).32 In this study, 660 adults with DMO were randomised to receive IVT-AFL 2.0 mg, IVR 0.3 mg or IVB 1.25 mg. Over 52 weeks, IVT-AFL produced a statistically significant two-letter BCVA benefit over IVR (13.3 versus 11.2 mean letter gain; p=0.03) and a statistically significant >3 letter BCVA benefit over IVB (13.3 versus 9.7 mean letter gain; p<0.001). These BCVA gains were achieved with a median of nine IVT-AFL injections versus 10 IVR and 10 IVB injections. Moreover, a significantly smaller proportion of patients receiving IVT-AFL required laser photocoagulation (at least once between 24 and 48 weeks) versus those receiving IVR or IVB (37 % versus 46 % or 56 %; p<0.001 for overall comparison).
The benefits of IVT-AFL in the Protocol T study were primarily driven by the patients with worse visual acuity at baseline.32 In the subgroup with better baseline vision (initial visual acuity letter score 78–69 or approximately 20/32–20/40), the mean improvement was 8.0 with IVT-AFL, 8.3 with IVR and 7.5 with IVB (p>0.50 for each pairwise comparison). However, in the subgroup with worse baseline vision (initial letter score less than 69 or approximately 20/50 or worse), IVT-AFL produced statistically significant and clinically meaningful benefits of approximately five letters over IVR (18.9 versus 14.2 mean letter gain; p=0.003) and approximately seven letters over IVB (18.9 versus 11.8 mean letter gain; p<0.001). Of note, a 15-letter gain was observed in 34 % more IVT-AFL-treated eyes than IVR-treated eyes
(67 % versus 50 %) and 63 % more IVT-AFL-treated eyes than IVBtreated eyes (67 % versus 41 %). In addition, among the subgroup with worse baseline vision, visual acuity benefits of IVT-AFL over IVR and IVB were demonstrated as early as 4 weeks after treatment initiation. Improvements in terms of decreased CRT mirrored those observed for visual acuity outcomes, with IVT-AFL showing greatest benefits over IVR and IVB among the subgroup with worse baseline vision.32 When considering the results of the Protocol T study, it should be noted that although the DRCR.net trial used the 0.3 mg dose of IVR (not the 0.5 mg dose that is approved in Europe), previous evidence suggests that there is no meaningful difference in efficacy outcomes between the two IVR doses.33
Safety
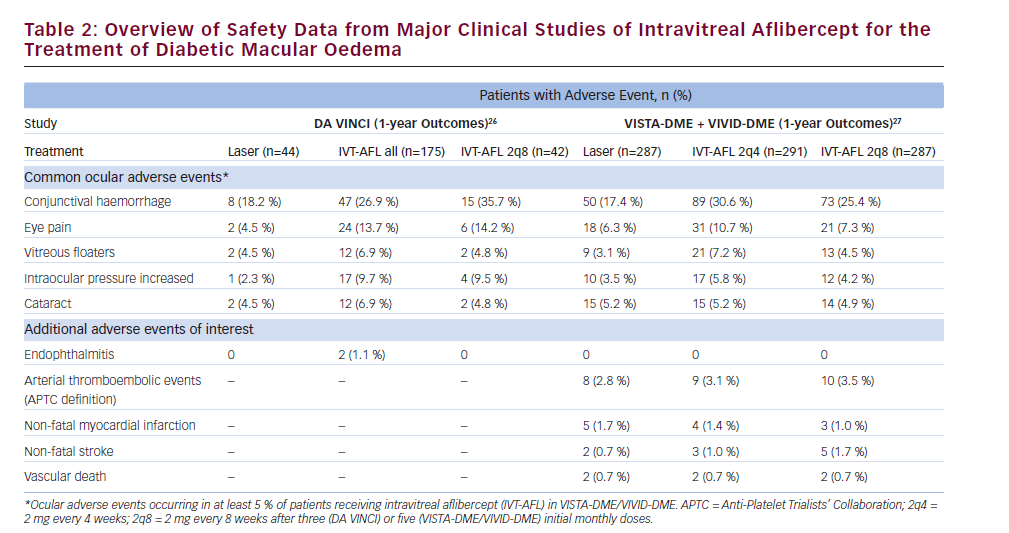
An overview of 1-year safety data from the DA VINCI, VISTA-DME and VIVID-DME studies is presented in Table 2. The overall incidence of ocular adverse events (AEs) in the VISTA-DME and VIVID-DME studies was similar across treatment groups, and no clinically relevant differences were observed in the frequency or pattern of serious ocular AEs. The incidence of endophthalmitis – a potential complication of intravitreal injections34 – was low, with only two cases reported across the study programme (a third patient in the DA VINCI study25,26 developed uveitis). Intraocular inflammation was also infrequent, occurring in 0.1–0.4 % of intravitreal injections in the first 52 weeks of the VISTA-DME and VIVIDDME studies.27
Intraocular steroids have been associated with an increased risk of cataract and increased intraocular pressure.12,13 Cataracts occurred at a similar rate in patients receiving IVT-AFL and those receiving laser treatment in the first 52 weeks of the VISTA-DME and VIVID-DME studies.27 In the DA VINCI study, increases in intraocular pressure occurred in 9.7 % of patients receiving IVT-AFL compared with 2.3 % of patients receiving laser treatment; however, these events occurred immediately after injection of IVT-AFL and normalised within 1 hour.25 Although interstudy comparisons should be interpreted with caution, the rates of increased intraocular pressure in studies of IVT-AFL (see Table 2) were lower than those reported in a systematic review of randomised clinical trials of intraocular steroids (injected or implanted) for DMO.35 The review reported that increased intraocular pressure occurred in 12–65 % of treated eyes (versus 0–6 % of control groups), while 0–43 % of treated eyes had cataract progression (versus 0–14 % of controls). Increased intraocular pressure and cataract progression occurred in both steroid injection-treated and steroid implant-treated eyes.35
The possibility of systemic AEs is a concern associated with the use of intraocular anti-VEGF therapies.36 The DA VINCI study did not show any significant safety signals suggestive of an increased risk of systemic AEs in patients receiving IVT-AFL.25,26 The VISTA-DME and VIVID-DME studies also showed no clear trends between treatment groups in serious systemic AEs and arterial thromboembolic events defined by the Anti-Platelet Trialists’ Collaboration (APTC; see Table 2).27 In the recent DRCR.net comparative effectiveness study in patients with DMO (Protocol T), the rates of APTC-defined arterial thromboembolic events were uniformly low: 3 % in the IVT-AFL group, 5 % in the IVR group and 4 % in the IVB group. Overall rates of pre-specified systemic AEs were similar in the three treatment groups.32 However, the ability of these clinical trials to detect rare systemic AEs may be limited by their relatively small sample sizes; ongoing surveillance will shed further light on the safety profile of IVT-AFL.27
Conclusions
IVT-AFL is a recently approved anti-VEGF treatment option for patients with visual impairment due to DMO. It has a markedly higher affinity for VEGF than IVR and IVB, and it also targets PlGF, another member of the VEGF family that has been shown to contribute to angiogenesis. In contrast to IVR (which requires once-monthly dosing), IVT-AFL has been approved with a dosing regimen of one injection every 2 months; it may therefore reduce the number of injections and office visits required, thus reducing the burden of treatment for patients, physicians and the healthcare system.
In clinical trials, IVT-AFL improved visual acuity and CRT to a significantly greater extent than macular laser photocoagulation; it also produced improvements in the DRSS, suggesting regression of the underlying diabetic retinopathy. IVT-AFL was generally well tolerated: the rates of endophthalmitis and intraocular inflammation were low, and the rates of APTC-defined arterial thromboembolic events showed no clear trends between treatment groups. Increases in intraocular pressure in patients receiving IVT-AFL were less common than previously reported in patients receiving intraocular steroids (based on indirect study comparison) and were generally transient; the incidence of another steroid-related AE, cataract, showed no difference between treatment groups in the phase III trials of IVT-AFL.
Results from a recent meta-analysis and a recent direct comparative effectiveness study suggested that IVT-AFL may be superior to IVR and IVB in terms of the extent of visual and anatomical benefits. Overall, these findings highlight the role that IVT-AFL has to play in the treatment of patients with DMO.