Fabry disease (FD) is a rare, X-linked, lysosomal storage disorder with a reported incidence of 1:117,000 live births.1 However, due to the pan-ethnic nature of FD, reported incidence rates of 1:117,000 may underestimate the true prevalence of the disease, which have been recorded as high as 1:3,100 live births.2,3
FD is caused by mutations in the gene encoding the enzyme alphagalactosidase A (α-Gal A), resulting in an absence or deficiency of this lysosomal enzyme. Complete or partial enzymatic insufficiency of α-Gal A leads to the accumulation of the plasma membrane component glycosphingolipids, predominantly globotriaosylceramide (Gb3; also known as ceramidetrihexoside [CTH]).2 Gb3 accumulates in the lysosomes of multiple cell types including the endothelial and smooth muscle cells of the vascular system, as well as renal, cardiac and nerve cells. FD is therefore a multisystem disease affecting the brain, heart, eyes, kidneys and other organs, which can result in end-stage renal disease and life-threatening cardiovascular or cerebrovascular complications.4,5
The phenotype in affected males reflects the level of α-Gal A activity.6 Classic FD is a term reserved for males having absolutely no α-Gal A activity. Individuals with this phenotype will develop the full complement of clinical signs and symptoms with onset usually in childhood. Males with atypical manifestations of FD have partial α-Gal A activity. These patients may experience symptoms in childhood, but they do not manifest the full clinical spectrum of classic FD. Typically these patients develop late-onset cardiac and renal abnormalities, which are non-specific and are often indistinguishable from those occurring in the general population. These adult-onset cardiac and renal variants seem to be much more prevalent than the classic phenotype and may explain many cases of midlife-cardiac and renal disease. Female carriers of FD can manifest clinical features depending on the level of X-inactivation or Lyonisation and can be considered affected clinically with variable penetrance.7 Data from disease registries demonstrate that, in fact, female FD gene carriers have a significant risk for major organ involvement and should be regularly monitored for signs and symptoms of FD.
Ophthalmological Opportunities for Diagnosis
The diagnosis of FD is often missed or delayed as FD shows similarities to several other common medical conditions,8,9 in particular those patients with ‘cardiac’ and ‘renal’ variants, and the symptoms are nonspecific. A delay in diagnosis has significant implications for the patient as early diagnosis is vitally important for achieving best outcomes for patients.10 Detecting the ocular features of FD, which are asymptomatic, offers a vital opportunity to diagnose FD at any early stage and can be detected by a routine eye examination.11 Much of the pain, suffering and adverse impact of FD can be avoided if an alert eye care professional sees the patient at an early age, identifies the condition and makes the appropriate referral.12,13
There are several distinctive and unusual ophthalmic manifestations of FD, which appear early in the disease’s course.14 These ocular signs can be detected by examination procedures that are noninvasive, inexpensive and non-time-consuming. However, as eyesight does not tend to be impaired by FD,15 diagnosis is almost always opportunistic, e.g. during a routine eye check by an optometrist or ophthalmologist.
Ocular Manifestations of Fabry Disease
Corneal Verticillata
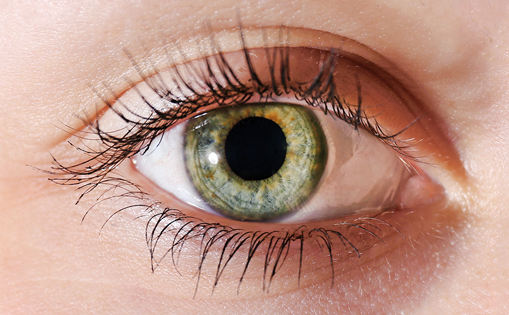
The accumulation of Gb3 in the cornea causes a typical but not pathognomonic FD lesion called cornea verticillata, which is considered to be the most reliable and common ophthalmological marker of FD. Gb3 is deposited by limbal blood vessels at the level of the epithelial basement membrane and results in the typical corneal appearance (see Figure 1), which can be detected by slit-lamp biomicroscopy.16,17 Corneal verticillata are present in approximately 77 % of females and 73 % of males (age range, 3–71 years) with FD.15 However, a few patients with a genetic, biochemical and clinical diagnosis of FD do not show cornea verticillata, even after several years. The signs of corneal verticillata usually begin in childhood as a diffuse haziness, which develops into the typical whorl-like rays of white, cream or brown-coloured inclusions that emanate radially from a single vortex (see Figure 1). The cause of the vortex pattern of the corneal deposits is unknown. Various theories cite the influence of ocular hydrodynamics, periodic blinking or ocular magnetic fields. Another possible cause is the centripetal movement of the renewing corneal epithelial cells from the periphery towards the centre of the cornea.18
Cornea verticillata is a key indicator of FD and should elicit further questioning of family history and medical evaluation. However, care should be exercised as a variety of drugs may result in corneal verticillata formation (see Table 1).19 Care must be taken as amiodarone used for cardiac disease may be prescribed in addition to an underlying diagnosis of FD.17 There is some evidence that in vivo confocal laser-scanning microscopy can detect differences between amiodarone-related corneal verticillata and FD.20 Other rare ocular conditions can result in vortextype lesions at the level of Bowman’s membrane (Melkersson-Rosenthal syndrome) or corneal stroma (Tangier disease). The detection of corneal verticillata if supported by clinical suspicion or family history should prompt clinical investigation.
Tortuous Vessels in the Conjunctiva and Retina
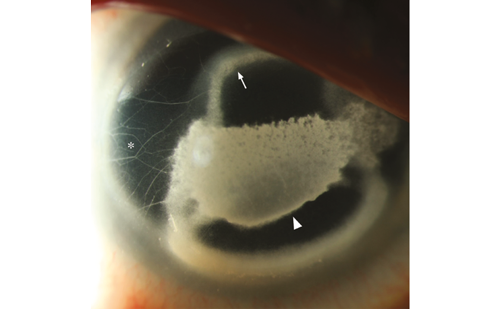
Gb3 accumulation in the vascular endothelium of the conjunctival, episcleral and retinal vessels (see Figure 2) results in vascular tortuosity. Vascular tortuosity is seen in the retina and conjunctiva, usually appearing in the second and third decade of life and has been reported in 22 % of females and 49 % of males (age range, 4–69).15 Retinal vascular tortuosity usually is seen in the venous circulation and in advanced disease the vessels have a ‘corkscrew’ appearance (see Figure 2). The retinal vascular changes are asymptomatic, however, one FD patient was reported with acute visual loss secondary to a central retinal artery occlusion.21 These vascular changes reflect the disruption of the systemic vascular system and can act as a predictor of more severe systemic involvement.
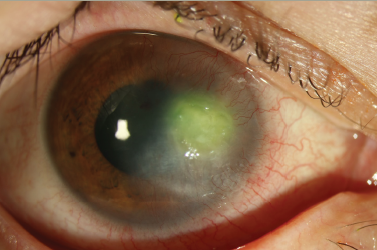
Lens Opacities and the Fabry Cataract
Gb3 deposits in the crystalline lens epithelium result in lenticular opacities, which are best seen on slit-lamp examination using retroillumination. Anterior and posterior subcapsular cataracts can be observed and frequently appear in the second decade of life. Linear, almost translucent spoke-like deposits on or near the posterior lens capsule, represent a pathognomonic ocular sign of FD and are termed the ‘Fabry cataract’. Fabry cataract is seen in 10 % of females and 23 % of males (age range, 5–68 years). The anterior cataract also has a characteristic appearance with wedge-shaped opacities distributed radially with their apices towards the centre of the anterior capsule (see Figure 3). They are seen in 70 % of males; however, this incidence is lower in females.6
Other Ocular and Visual Observations
A number of other ocular features have been described in FD. In almost 40 % of males with FD in one study there was an enlargement of the blind spot detected on visual field testing.22 There was no evidence of optic nerve dysfunction, however, the underlying pathophysiology mechanism was unclear. A variety of visual field defects can occur due to ischaemic events in the visual pathways.
A number of ocular surface and adnexal problems have been reported in FD. Dry eye syndrome resulting from decreased aqueous tear production was detected in approximately 50 % of FD patients.6 Vascular tortuosity of the upper eyelid has been reported at high prevalence; a recent study observed high levels (94.7 % of FD patients observed, n=36) of tortuous vessels in the upper eyelid. This may be a benign sign and could be taken into account when suspecting FD.23
Angiokeratoma, benign vascular skin lesions (see Figure 4), are seen in 83 % of males and 80 % of females with FD, first appearing in children between the ages of 5 to 15.24 Periorbital fullness, prominent supraorbital ridges, bushy eyebrows and bilateral ptosis can occur but are not prominent features of FD. Course craniofacial features can include a broad nasal base, full lips, prominent chin and earlobes and posteriorly rotated ears have been reported in FD.
Systemic Implications
Ophthalmic indications are common in the early stages of FD and are part of the wider systemic implications for this disease. FD manifestations typically develop at different age ranges as the disease progresses. Broadly described, FD symptoms can be expected to be seen in the following pattern (see Table 2).25
Not all patients will demonstrate this pattern and this can be particularly varied in heterozygous patients, who tend to have less severe symptoms than classic FD patients.10 In such patients cornea verticillata may be a prominent indicator of FD, occurring in approximately 77 % of people. Other symptoms to be aware of include angiokeratoma and pain in the extremities.
Referral Pathways
Ophthalmic markers should raise suspicion of FD, particularly if a family history of FD is established upon questioning or if corneal verticillata are unlikely to be drug induced (caution is recommended in patients using amiodarone given the cardiac features of FD). Upon identification of these markers the observing ophthalmologist has the opportunity for referral to a specialist for diagnosis and, if then considered appropriate, treatment with enzyme replacement therapy. Referral should be made to a Lysosomal Storage Disorder centre – a list can be found on the Focus on Fabry website (www.focusonfabry.com).
Diagnosis of Fabry Disease
Biochemical
α-galactosidase activity can be measured in plasma, serum, leukocytes, tissue biopsies and cultured skin fibroblasts. In males, the demonstration of a deficient activity of α-galactosidase activity in plasma or ideally leukocytes is used to confirm the clinical diagnosis of FD. In females, their α-galactosidase activity can fall within the normal range. Therefore, all females should have their disease status determined by genetic testing.26
Genetic
FD results from pathogenic defects in the α-galactosidase (GLA; MIM *300644) gene. GLA is located on chrXq221 and contains seven exons coding for a polypeptide of 429 amino acids. Over 600 mutations in GLA have been reported to result in FD and consist of a variety of pathogenic variants, including missense and nonsense mutations, large and small gene rearrangements and splicing defects.27 Genetic testing can be arranged through Clinical Genetics services.
Histology/Biopsy
Histopathological analysis of endomyocardial and kidney biopsies, using light and electron microscopy, can demonstrate characteristic undegraded intralysosomal Gb3 deposits commonly known as myelin bodies or zebra bodies.6
Conclusion
FD is a progressive disease and early diagnosis to minimise Gb3 accumulation is beneficial to patient outcomes.10 Effective management regimes for FD are available, with the long-term goal of prevention of irreversible damage to vital organs.
FD can be a severely debilitating disorder, affecting quality of life and often progressing undiagnosed for many years;8 late diagnosis can result in a significantly reduced life expectancy.28 A thorough ophthalmological examination provides a unique and important opportunity to diagnose FD early, ensuring patients are referred for management to prevent disease progression. Simple ophthalmic techniques and an alert and inquisitive ophthalmologist/optometrist offer a major opportunity in the successful diagnosis of this disease.
Appendix/Additional Notes
Management for specific systemic manifestations of FD should be considered for all cases and a few treatment options are noted in Table 3.29
FD should be considered in the differential diagnosis of fever, pain and skin lesions of unknown origin, or in stroke or renal disease of unknown aetiology. Additionally, symptoms in FD are similar to those of other disorders (see Table 4).7