Descemet’s stripping automated endothelial keratoplasty (DSAEK) has become the procedure of choice to treat corneal endothelial dysfunction. Developed by Melles et al.1 and modified by others,2 DSAEK evolved from posterior lamellar keratoplasty (PLK) and deep lamellar endothelial keratoplasty (DLEK). The host Descemet’s membrane is removed and a graft consisting of posterior stroma, Descemet’s membrane and endothelial layer is inserted.3 Initially, the grafts were manually dissected and known as Descemet’s stripping endothelial keratoplasty (DSEK). Most grafts are now pre-dissected with microkeratomes. DSAEK is preferred over penetrating keratoplasty (PK) in treating corneal decompensation from endothelial disease as it confers quicker visual and structural recovery with absence of corneal surface incisions or sutures, and also limits astigmatism.4 With the advent of Descemet’s membrane endothelial keratoplasty (DMEK), in which only Descemet’s membrane is transplanted, visual rehabilitation may be attained earlier.
Indications
DSEK/DSAEK has been described to successfully achieve favourable visual acuity and graft clarity in bullous keratopathy5 secondary to Fuchs’ dystrophy, cataract surgery,6 posterior polymorphous dystrophy, iridocorneal endothelial syndrome (ICE syndrome)7 and failed PK grafts.8 Although visual rehabilitation after DSAEK is quicker than after PK, endothelial cell loss is higher (20–57% at six-month and one-year follow-up).3,6,8–16 Various studies reported best corrected visual acuity (BCVA) ≥20/40 (logMAR 0.3) ranging from 28 to 100% atsix months6,10,12,14,15,17 and from 66 to 100% at one year.3,8,11,18 BCVA ≥20/25 (0.1) was also noted to range from 0 to 20% at both six months6,10,12,14,15,17 and one year.3,18
Technique
Donor Dissection
In DSAEK, the graft is dissected with a microkeratome. In a randomised, prospective, double-masked clinical trial, Price et al. found that manually dissected grafts and eye-bank pre-cut grafts possessed similar endothelial cell loss, visual and refractive outcomes and detachment rates.14 Donor lenticule thickness <350μm measured with slit-lamp optical coherence tomography (SL-OCT) one week after transplantation correlated with >98% success in achieving anatomically attached, clear recipient corneal stroma and donor lenticule compatible with good vision two months after surgery.19
Similarly, Terry et al. found that pre-cut tissue was comparable to donor tissue cut intraoperatively. At six- and 12-month follow-up, best spectacle-corrected visual acuity (BSCVA), refractive astigmatism and pachymetry were not statistically significantly different between the two preparations. Visual improvement was not significantly influenced by donor thickness, death to preservation time, death to surgery time or eye-bank cutting time to surgery time.15 Furthermore, an eye-bank survey of surgeons showed that pre-cut tissue was not associated with an increased risk of complications. Rather, success rates have been correlated with surgeon experience, lenticule adherence after injection of an anterior chamber air bubble, lack of venting incisions to release interface fluid and presence of corneal deturgescence.20,21
Storage of grafts with anterior lamellar corneal tissue (ALCT) on corneas had less endothelial damage and oedema than those stored without the stroma for 24 hours in Optisol GS. This is thought to be due to the Bowman layer barrier preventing oedema and a loose Descemet’s membrane that could easily detach from stroma.22 Central anterior cap thickness of pre-cut grafts were compared and found to be dependent on depth plate, eye bank and pre-dissection thickness. As with laser in situ keratomileusis (LASIK) flap thickness, thicker corneas led to deeper cuts.23 Thickness and curvature coefficients derived from the graft thickness profile are known to alter refractive shift.24
Femtosecond laser is another modality used to cut DSAEK grafts. The laser performed deep posterior stromal ablations to achieve accurate, intended measurements of corneal thickness and diameter of corneal buttons in an ex vivo study.25 Femtosecond-cut grafts are purported to produce minimal change in refractive astigmatism and cause a mild hyperopic shift in refraction.26,27
Surgical Technique and Donor Positioning
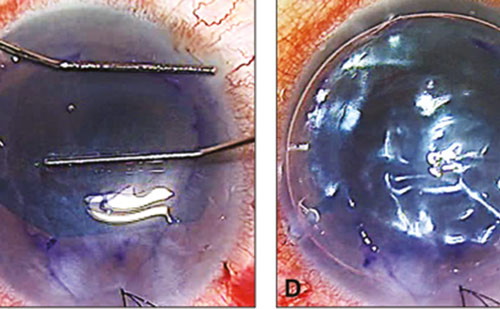
In DSAEK, the diseased endothelium is replaced by a graft consisting of a thin layer of posterior stroma, Descemet’s membrane and endothelium.28 Atraumatic graft insertion is one of the main challenges. The techniques involve pushing or pulling the graft into the anterior chamber. Initially, graft insertion was performed using McPherson forceps to position a folded donor posterior corneal disc into a tacolike formation over a plastic glide into the recipient anterior chamber.29 Busin described a technique in which the donor tissue is pulled into the anterior chamber with a microincision forceps through the opposite limbus to reduce trauma to the graft and limit endothelial loss compared with forceps insertion.9,10 Using a 10-0 monofilament suture on a long straight needle to pull the graft through is another technique that was found to have similar post-operative visual acuity, complications and endothelial cell counts to forceps-assisted DSAEK.11,12,30 Needle graft insertion techniques are also cost-effective.28 Intraocular lens (IOL) cartridges have also been used in ex vivo studies, where the graft is ‘rolled’ into a compact shape to avoid compressive, deleterious forces that occur as a result of folding.31,32 Modifications of the glide and forceps techniques to reduce endothelial injury during insertion are currently under investigation.29,33
Roughening the host peripheral stromal edges has demonstrated better graft adherence.34 Pre-soaking the grafts in balanced salt solution (BSS) Plus also significantly reduced graft detachment rates in DSEK patients.35 LASIK rollers have been used to centre donor graftsand remove interface fluid.36 Others have shown that air–fluid exchange can be used to control anterior chamber pressure and effectively tamponade the graft against the host stroma.37
Combined Procedures
Combined procedures or ‘triple procedures’ include concomitant cataract extraction and IOL implantation with DSAEK.38 IOL calculations can be performed with consideration of expected hyperopic shifts (1.25–1.50D) that occur with DSAEK. In Fuchs’ dystrophy patients, statistical comparison of post-operative visual acuity showed no significant difference between those who underwent DSAEK only and those who had a triple procedure. The DSAEK-only group showed a larger hyperopic shift from the pre-operative spherical equivalent than the triple procedure group. No ignificant difference in endothelial cell loss was noted between the two groups. Iatrogenic graft failure, or primary graft failure, was defined as persistent post-operative corneal oedema that failed to clear within two months in a well-apposed graft; this was not observed in either of the groups.39
Case reports of a triple procedure in Fuchs’ dystrophy patients involving anterior chamber IOL implantation have been successful in patients with an anterior chamber depth >3mm.40
New Surgeons
New surgeons are encouraged to start performing DSAEK on patients who have an intact iris–lens diaphragm in order to prevent donor dislocation.41 There is a relatively steep learning curve to DSEK/DSAEK; however, a recent study comparing surgical results from attendings and fellows showed that vision, endothelial loss and complicationswere not statistically different at six-month follow-up.17
Comparisons
Comparisons between DSAEK and other types of keratoplasty have been favourable. In comparing PK and DSAEK, BCVA ≥20/40 (0.3) was 70–80% in DSAEK and 25% in PK patients, and ≥20/25 (0.1) in 6–20% in DSAEK and 0% in PK patients at one-year follow-up.15,18 Although PK is known to have a longer course of visual rehabilitation, BCVA of DSAEK at one-year follow-up was superior to that of PK at two- to three-year follow-up: PK patients ≥20/40 (0.3) was 55% and ≥20/25 (0.1) was 20%.18 However, primary graft failure was more common in DSAEK at one-year follow-up.13,18 This may be attributed to surgical technique. In a patient survey comparing deep lamellar endothelial keratoplasty (DLEK) and DSAEK, perception of quicker visual recovery was higher with DSAEK even though post-operative visual acuity was not significantly different between the two groups. This may be attributed to fewer higher-order aberrations and limitations of surgery-induced hyperopia in DSAEK.42 There was no statistically significant difference in endothelial cell loss between DSEK and DSAEK at six or 12 months.16
Case reports of DSAEK in children have shown a more predictable refractive outcome with less astigmatism and quicker recovery – crucial for amblyopia therapy – in comparison with PK.43–45 Structural integrity is also enhanced due to absence of sutures between thehost and graft in DSAEK.
Complications
Graft dislocation is the most common complication in DSAEK, ranging from 1 to 50% in the literature,3,6,8,10–15,34,46,47 and may be related to proper positioning of the graft with appropriate pressurisation to maintain apposition. Terry et al. reported that roughening the peripheral edges of the host stroma decreased the graft dislocation rate to 4%. Post-operative re-positioning or re-injecting an air bubble (re-bubbling) can achieve graft re-attachment in most cases.46 A slit-lamp technique of draining fluid at the wound interface successfully re-attached the donor disc in five cases.48
Primary graft failure ranges from 0.5 to 5%,49 with loss of endothelium as the predominant factor in histopathological evaluation of grafts.50,51 As stated earlier, endothelial cell loss ranged from 20 to 57% in several studies.3,6,8–16 This may be explained by many factors, including surgical experience, inadvertent epithelial implantation during lenticule preparation, retained Descemet’s membrane in the graft, epithelial ingrowth, fluid or material accumulation at the wound interface (viscoelastic, neovascularisation, calcification),52,53 de-centred grafts with full-thickness corneal layers at one edge or chronic stromal changes from chronic corneal oedema.54,55 Cases of epithelial downgrowth have been treated successfully with lenticule exchange, mechanical scraping and irrigation and aspiration of residual epithelial cells.56–58
Other complications include pupillary block, steroid-induced glaucoma, suprachoroidal haemorrhage, infectious donor-to-host transmission of fungal pathogen, cystoid macular oedema and retinal detachment. Air bubble trauma to endothelial cells has also been described in an ex vivo study.59
Future
DMEK is currently under investigation.60 This procedure involves implantation of isolated Descemet’s membrane and endothelial cells in the host cornea through a self-sealing 3.5mm clear corneal incision.
A study of 50 DMEK procedures by Ham et al. conveyed promising results: >95% had BCVA better than 20/40 (≥0.5) and 75% had BCVA better than 20/25 (≥0.8). They also showed faster visual rehabilitation (within one to three months) and less refractive shift.61 As these procedures are refined with innovative advances in tissue handling, the sole replacement of cultured endothelial cells with faster visual rehabilitation may be in the near future.