Medical treatment – keeping 24-hour intraocular pressure control while maintaining conjunctival integrity
Intraocular pressure (IOP) is the main risk factor for the development and progression of glaucoma.1,2 The Ocular Hypertension Treatment Study (OHTS), Collaborative Normal Tension Glaucoma Study (CNTGS), United Kingdom Glaucoma Treatment Study (UKGTS) and Early Manifest Glaucoma Trial (EMGT) all demonstrated longer times to progression of open-angle glaucoma with effective IOP-lowering eye drops.3,4,5,6 However, none of these treatments could completely prevent glaucoma from developing or worsening, suggesting other factors are at play.3,4,5,6
The IOP fluctuations occur within and between days, and the threshold for IOP-induced damage can vary within and between patients, as glaucoma progresses.7 There is evidence that diurnal fluctuations in IOP are greater in glaucomatous eyes than normal eyes.8 As a result, it is now recognised that IOP values observed in the clinic may underestimate the true measure of IOP fluctuations over 24 hours,9 leading to missed diagnoses, underestimation of the impact and severity of the disease and thus a general misconception that patients are well controlled on their prescribed medication.7
There have been limited data on how different IOP-lowering eye drops work over a 24 hour-period. In studies where this has been investigated, untreated patients with early glaucomatous changes have been shown to have higher diurnal IOP compared with healthy eyes.10 Wide diurnal ranges in IOP can have significant health consequences, with up to six times the relative risk of disease progression versus low fluctuations, despite adjusting for well-known risk factors for glaucoma progression.11
To date, only one meta-analysis has addressed the 24-hour efficacy of glaucoma treatments, including fixed combination IOP-lowering medications in glaucoma.12 This analysis comprises 11 studies including just 386 patients across 28 treatment arms.12 With these limited data, prostaglandins have been generally shown to provide peak
IOP-lowering efficacy around 8–12 hours after administration, with an ocular hypotensive effect that appears to be fairly uniform throughout the circadian cycle.12,13 This also seems to be the case for carbonic anhydrase inhibitors.13 However, with beta-blockers, the efficacy observed overnight was about half that seen during the day, suggesting these are less efficacious over the 24-hour period than prostaglandins and carbonic anhydrase inhibitors.13
When choosing topical therapy for effective IOP reduction in patients with glaucoma, it is also important to consider the role of preservatives. Although benzalkonium chloride (BAK) provides broad antimicrobial efficacy, it is associated with corneal epithelial breakdown and tear film instability.14 The SofZia® Preservative System, TravatanZ® 0.004% (Alcon Laboratories, Inc., Fort Worth, TX, US) and Purite® (Bio-Cide International Inc., Oklahoma city, OK, US) preservative are associated with less conjunctival inflammation and corneal toxicity than BAK, but mild cytotoxicity may still present.14
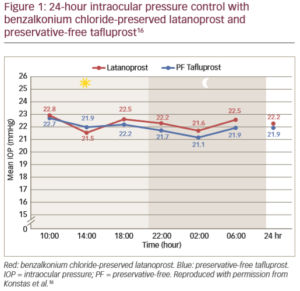
When preservative-free eye drops were compared with preserved prostaglandin eye drops for their effect in glaucomatous eyes, it was found that there was a significant increase in tear inflammatory cytokine levels with the preserved drops versus normal controls, but not for the preservative-free drops.15 Both apoptosis and inflammation participate in ocular adverse events associated with the use of preservatives in ophthalmic preparations.15 This can impact on clinical outcomes, evidenced by statistically greater 24-hour efficacy and improved tolerability for preservative-free tafluprost therapy compared with preserved latanoprost (Figure 1).16 Preservatives are also known to have a negative effect on the success of future glaucoma surgery.15
It is now recognised that, unless IOP is assessed over a 24-hour period, IOP peaks – which are an important risk factor of glaucoma progression – will be inadequately assessed.7 However, 24-hour monitoring is
time- and resource-consuming and rarely feasible in routine clinical practice.7 Therefore, it is important to choose the optimal topical therapy that ensures sustained 24-hour IOP control.16 On the other hand, it
has to be recognised that some patients are inadequately treated with topical medications, resulting in progression of disease despite therapy. In this case, other treatment options need to be considered.7
Surgical management of glaucoma – where are we today?
The goal of IOP reduction in glaucoma should be to reduce the risk of blindness, resulting in an improved patient quality of life, as well as reducing the treatment burden.17 However, glaucoma progresses in the majority of people diagnosed with the disease, despite treatment.18 As a result, glaucoma remains a leading cause of blindness, with a lifetime risk of around 40% for blindness in one eye and 16% for bilateral blindness.19,20
Trabeculectomy has been considered the gold standard in surgical management of glaucoma for many years.21 However, trabeculectomy is a technically complex procedure that may result in a range of adverse outcomes and can often require repeat procedures.21,22 In the last
20 years, the number of trabeculectomies for treating glaucoma have been on the decline, while the use of mini-shunts has been increasing, albeit slowly.23
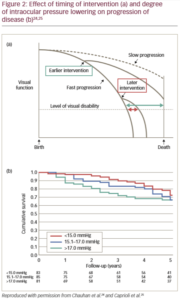
Early and aggressive lowering of IOP for glaucoma has been shown to slow down the rate of progression of disease (Figure 2).24,25 However, clinicians can be reluctant to take this approach when there’s a high risk of complications from trabeculectomy.21,22 Therefore, it may be time to consider a new treatment approach in early stage glaucoma, with more aggressive interventional approaches aimed at maintaining low IOP from the start, versus the established step-wise approach with therapy escalation and initial modest IOP targets that are associated with continued disease.
Aggressive therapy with the addition of a third and fourth anti-glaucoma medication produces a clinically significant reduction in IOP in about 40–60% of patients at any single time point.26 However, the cumulative probability of success including safety outcomes is relatively poor at 6 months and 1 year, suggesting that adding another anti-glaucoma medication to a regimen of two or three medications frequently does not achieve a significant (≥20%) fall in IOP.26 An additional factor to consider is the issue of compliance with medication, which remains a major unmet need in glaucoma treatment and correlates with disease progression, which in turn increases the cost of glaucoma care.27–9
A Cochrane review states that primary surgery lowers IOP more than primary medication.30 However, it may be associated with more eye discomfort, and the clinical and cost-effectiveness of surgery versus contemporary medication has yet to be determined.30 The efficacy of glaucoma surgery has been investigated in patients as a primary procedure and in those who previously received IOP-lowering medical therapy.31 Increased preoperative exposure to ophthalmic solutions preserved with BAK was shown to be a risk factor for earlier surgical failure, independent of the number of medications used.31 When directly compared, the success rate of trabeculectomy was significantly higher for primary trabeculectomy versus subsequent procedures (p<0.001).32
Compared with medical treatment, patients treated with trabeculectomy surgery have shown lower mean diurnal IOP, lower peak IOP and less IOP fluctuation with fewer spikes.33,34 The aim of minimally (or micro) invasive glaucoma surgery (MIGS) is to achieve a similar efficacy to that of trabeculectomy, but provide a better risk/benefit outcome and offer a less invasive technique for IOP reduction.21,22
Five distinct qualities are associated with MIGS:
- microincisional, conjunctiva-sparing approach;
- minimal trauma to and disruption of normal anatomy and physiology, with devices that exhibit a high level of biocompatibility;
- moderate to high IOP-lowering efficacy;
- a positive safety profile; and
- rapid recovery by the patient.35
These surgical techniques have sought to improve upon existing surgical approaches and currently include three main aqueous outflow targets: the Schlemm’s canal, the suprachoroidal space or the subconjunctival space.36 The effectiveness of any MIGS device is dependent on its implantation site, composition and design.21
Several procedures have been developed that either remove or bypass the juxtacanalicular trabecular meshwork and inner wall of Schlemm’s canal, allowing aqueous direct access to the low resistance collector channels in the outer wall of Schlemm’s canal.36 Suprachoroidal drainage devices aim at a potential space where IOP reduction is not limited by episcleral venous pressure and bleb formation is not required.36 However, scarring in the suprachoroidal spaces remains an issue.36 In addition, these ab interno approaches remain invasive and intraocular.21
The subconjunctival space is the traditional target for glaucoma drainage surgery and, as a result, less invasive procedures have been developed that create a new outflow pathway, allowing aqueous to enter the subconjunctival space and resulting in bleb formation.36 The subconjunctival drainage of aqueous humour has been the cornerstone of glaucoma surgery, and new devices and procedures targeting the subconjunctival space appear to be more efficacious in lowering IOP than those targeting the Schlemm’s canal or suprachoroidal space.22,36
New devices should be simple for surgeons to use and provide a sustained reduction in IOP, with postoperative management suitable for the general ophthalmologist and with few potential complications.21 The use of MIGS has already demonstrated a number of potential advantages over other medical and surgical strategies, including reducing the medication burden, which enhances patient quality of life, bypassing or delaying the need for more invasive surgery and preserving the conjunctiva if more invasive interventions are required at a later date.22
The path to predictable and sustainable surgical results
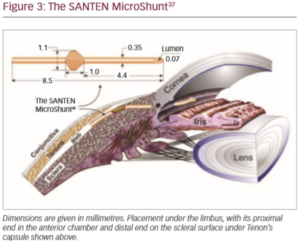
The novel glaucoma drainage implant (the Santen MicroShunt; Santen Pharmaceutical Co., Ltd., Osaka, Japan) was developed in response to the limitations and challenges of trabeculectomy and MIGS. This device – which is made from poly(styrene-block-isobutylene-block-styrene) or ‘SIBS’ – has shown promise in clinical trials (Figure 3).37,38 The use of SIBS provokes clinically insignificant inflammation and encapsulation, its flexibility allows it to conform to the curvature of the eye and its composition makes it long-lasting.38 It is also tough, which enables the bleb to be repaired or the device to be cleared with a laser without damage, if required.38 Finally, the device is sufficiently small, allowing other procedures in the same eye in the event of bleb failure.38 Compared with trabeculectomy, implantation of the device is a straightforward and relatively quick procedure. The device is implanted via an ab externo approach with conjunctival and Tenon dissection and obviates the need for the scleral flap, sclerostomy, iridotomy and tensioning sutures used in trabeculectomy.37
A 6–8 mm wide incision with a deep sub-Tenon pocket and a large area of conjunctival dissection facilitates the formation of a large diffuse bleb that extends posteriorly.37 Inserting the fins of the device securely into the scleral pocket helps avoid peritubular flow and minimises the occurrence of postoperative hypotony.37,38 Flow of aqueous humour can be confirmed by observing drop formation at the distal end of the tube.37 At the end of surgery, the distal end of the tube should be checked with visualisation or for free mobility under the conjunctiva to ensure that it is not occluded by the Tenon capsule when it is tucked underneath the capsule. Careful conjunctival closure will help prevent postoperative bleb leaks.
A 3-year observational study investigating this first minimally invasive stand-alone procedure in mild, moderate and severe stage open-angle glaucoma has shown sustained lowering of IOP to values <15 mmHg, representing a durable reduction of 50% and the potential to eliminate eye drop medications in the majority of patients with glaucoma.37 The final phase of a randomised clinical study (NCT01881425) directly comparing the drainage implant device to trabeculectomy is underway in 29 centres across the US and Europe.39 ❒
Take home messages
- The treatment of glaucoma includes both medical and surgical approaches.
- More emphasis is now being placed on minimally (or micro) invasive glaucoma surgery (MIGS).
- Intraocular pressure (IOP) fluctuates throughout the 24-hour daily period and as a result, isolated measurements can overestimate the efficacy of topical IOP-lowering medication, leading to inadequate treatment, which in turn can result in disease progression. However, it is often difficult to monitor 24-hour IOP in a normal clinical setting.
- It is important to take 24-hour IOP-lowering efficacy into consideration when choosing medical therapy for glaucoma.
- Trabeculectomy has been considered the gold standard in surgical management of glaucoma, but is a technically complex procedure that may result in a range of adverse outcomes and can often require repeat procedures.
- MIGS include three main aqueous outflow targets: the Schlemm’s canal, the suprachoroidal space or the subconjunctival space.
- MIGS is minimally traumatic, with a high level of safety and rapid recovery and may be associated with better risk/benefit outcomes and offers a less invasive technique for lowering IOP compared with trabeculectomy.
- MIGS devices targeting the subconjunctival space appear to be more efficacious in lowering IOP than those targeting the Schlemm’s canal or suprachoroidal space.
- A novel glaucoma drainage implant (Santen MicroShunt) made from poly(styrene-block-isobutylene-block-styrene), which is inserted in a straightforward and relatively quick procedure compared with trabeculectomy, has shown promise in a 3-year clinical trial in various stages open-angle glaucoma.







