Thyroid eye disease (TED) is an autoimmune condition that can lead to significant visual impairment, facial disfigurement and decreased quality of life. It affects 16 out of every 100,000 females and 2.9 out of every 100,000 males. Risk factors for TED include age, female gender, smoking and radioactive iodine therapy.1 TED typically begins with a progressive inflammatory phase (previously termed ‘active’) that can last from 6 to 36 months. Common symptoms include dry eyes, periorbital pain and swelling and eyelid retraction. Some patients may develop disfiguring proptosis, diplopia and vision loss. Over time, patients enter a non-progressive chronic phase (previously termed ‘inactive’) characterized by fibrosis. An accurate diagnosis is based on clinical assessment of disease activity and severity, laboratory testing for dysthyroid states and orbital imaging.2
Targeted therapies have changed the treatment landscape for TED. Historically, corticosteroids and/or orbital radiation therapy were used to decrease inflammation in the active phase of the disease followed by surgical rehabilitation once the patient entered the non-progressive chronic phase. In January 2020, teprotumumab, an antibody targeted against the insulin-like growth factor-1 receptor (IGF-1R), became the first US Food and Drug Administration (FDA)-approved therapy for TED.3 Clinical trials demonstrated that teprotumumab therapy improves the clinical activity score (CAS), proptosis, diplopia and quality of life for patients with TED.4 Following the success of teprotumumab, several other investigational drugs are currently in development. With the advent of new therapies, it is important to understand each drug’s mechanism of action, safety profile and efficacy to safely and effectively customize our treatments for patients with TED.
Pathophysiology
TED is characterized by autoinflammation resulting from complex and incompletely understood mechanisms. Several processes contributing to TED have been identified, including cellular immunity, cytokine upregulation, glycosaminoglycan (GAG) synthesis and adipogenesis.5–10 Exploration of potential molecular mediators of TED has revealed that the insulin-like growth factor-1 (IGF-1) pathway is implicated in many autoimmune diseases, including TED.11,12 IGF-1 is a cell surface protein widely expressed in most tissues in the human body. The IGF-1 pathway acts synergistically with thyroid-stimulating hormone (TSH), and once activated, the thyroid-stimulating hormone receptor (TSHR)–IGF-1R complex leads to the production of cytokines and expression of genes that result in orbital tissue reactivity and remodelling.10,13–15 The cytokine released recruits inflammatory cells into the orbit and stimulates fibroblasts to secrete GAGs, resulting in adipose and muscle expansion.16 The activated fibroblasts can also differentiate into adipocytes and myofibroblasts, further expanding orbital tissue volume and fibrosis.16
Additionally, B and T lymphocytes have been implicated in the pathophysiology of TED. Antigen-presenting cells present the autoantigen to CD4+ helper T cells, leading to T-cell activation and proliferation. The T cells then may induce B cells to produce more antibodies against the autoantigen.17–21 CD20 is a surface antigen involved in B-cell maturation, which helps in antigen presentation and cytokine production.17 The cycle continues as memory B cells are involved in autoantibody production, which then leads to cellular dysfunction and inflammation.18 T cells may also directly lead to autoreactivity. Activated T cells may express CD145, which binds to CD40, leading to stimulation of both orbital fibroblasts and T cells.19 This, in turn, leads to cellular proliferation, GAG secretion and production of proinflammatory cytokines, including interleukin-6 (IL-6) and IL-8.19,20 T-helper 1 (Th1) cells dominate in the early phase, with a cytokine expression profile consisting of IL-2, interferon-γ and tumour necrosis factor-α (TNF-α).21 Later in disease, Th2 cytokines, such as IL-4, IL-6, IL-10 and IL-13, predominate.9,18,22,23 Cytokine release leads to the activation of the CD40:CD154 signalling pathway in orbital fibroblasts and B-cell proliferation, increasing autoantibody production.21,24,25 IL-6 can also promote adipogenesis and increase the expression of TSHR on orbital fibroblasts.26 Further studies into the signalling networks and molecular triggers involved in TED pathogenesis will help to improve our understanding of TED and direct future therapeutic development.
Medical management for progressive inflammatory (active) thyroid eye disease
Mild thyroid eye disease
Treatment for TED depends on the patient’s disease activity and severity.27 Mild TED can be managed with conservative intervention. Treatment involves optimization of the ocular surface with lubricants, management of risk factors that can exacerbate TED and thyroid hormone control under the guidance of the endocrinologist. Modifiable risk factors for TED progression include tobacco use, radioactive iodine treatment, high total cholesterol and low-density lipoprotein levels and vitamin D deficiency.28–30 A randomized clinical trial (RCT) found that the antioxidant, selenium, is associated with decreased soft-tissue inflammation and slowed the progression of disease when given at a dose of 100 μg twice a day for 6 months.31 However, the study enrolled patients with TED from selenium-deficient areas. In areas without selenium deficiency, such as the USA, this therapy may not have the same beneficial effects.
Moderate-to-severe thyroid eye disease
Corticosteroids
Historically, the first-line treatment option for active, moderate-to-severe TED was corticosteroids.32 Intravenous (IV) corticosteroids are more effective and better tolerated than oral corticosteroids.33,34 Patients with TED treated with methylprednisolone IV 500 mg weekly for six doses followed by 250 mg weekly for six doses achieved a response rate of 77% versus 51% of those treated with oral prednisone.33 An investigation of the optimal cumulative dosing for IV corticosteroid therapy found that improvement was more common with higher-dose regimens (52% in the 7.47 g group, 35% in the 4.98 g group and 28% in the 2.25 g group – doses were selected by the authors as amounts that resulted in efficacy while still safely below the threshold to cause hepatotoxicity).35 However, these differences were lost by 24 weeks, and major adverse events were slightly more common in the highest dose group.35 Studies have not reported a significant improvement in proptosis or diplopia with corticosteroid therapy. Patients receiving a cumulative dose greater than 8 g of methylprednisolone or equivalent may develop hepatotoxicity, which is a potentially fatal complication.36 Contraindications to steroid therapy include liver dysfunction, recent hepatitis, severe hypertension, cardiovascular disease, poorly controlled diabetes and severe steroid-responsive glaucoma.32
Orbital radiation
Orbital radiotherapy (ORT) presents another therapeutic option for active TED. Orbital lymphocytes have been found to be radio-sensitive, resulting in reduced inflammation.37,38 Proper patient selection is critical, as patients with early and progressing, active, moderate-to-severe TED show the highest response rates.39 Two RCTs comparing ORT with sham irradiation in moderate-to-severe TED demonstrated a response rate in a composite ophthalmic score of 50–60%.40,41 The main outcome improved was the diplopia score, with no significant effect on proptosis, CAS or eyelid aperture found.40,41 Potential side effects of ORT include conjunctivitis, dry eye, cataract formation and radiation retinopathy. Contraindications to ORT include diabetic retinopathy, severe hypertension and age under 35 years.38,42
Additionally, treatment with a combination of radiotherapy and corticosteroids can be more effective than either treatment alone.34,43 Concurrent ORT and corticosteroid therapy have been found to improve clinical scores, decrease the cumulative dose of steroids needed and reduce the overall length of therapy when compared with sequential treatment in moderate-to-severe TED.44 A prospective randomized study showed that ORT with oral prednisone (100 mg/day for 7 days, followed by 5–6 month taper) was more effective than ORT alone in active TED.43 ORT with IV steroids was also found to be more effective than ORT with oral steroids. In a study comparing ORT with oral prednisone (100 mg/day followed by 5-month taper) and ORT with methylprednisolone IV (15 mg/kg for four cycles followed by 7.5 mg/kg for four cycles) in active, moderate-to-severe TED, the ORT with IV steroid group resulted in a greater improvement in CAS (2.8 versus 2), fewer surgical procedures at follow-up (7 versus 22%) and lower rates of adverse events (56.1 versus 85.4%).45
Mycophenolate mofetil
Mycophenolate mofetil (MMF) has been used to treat TED by inhibiting the proliferation of T and B lymphocytes involved in inflammation.46 An RCT comparing MMF alone (500 mg twice a day for 24 weeks) with corticosteroids (methylprednisolone IV 0.5 g a day, 3 days a week, followed by an oral prednisone taper) in active, moderate-to-severe TED found that the MMF group demonstrated better CAS, diplopia and proptosis responses at 24 weeks, as well as a lower rate of disease reactivation on follow-up.46 MMF is commonly used in combination with other treatments for TED, in particular corticosteroids. The MINGO (Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy; EUDRACT number: 2008-002123-93) study, a multicentre, randomized observer-masked trial, found that the addition of MMF 360 mg twice a day for 24 weeks to methylprednisolone IV (500 mg/week for 6 weeks followed by 250 mg/week for 6 weeks) is superior to steroids alone in improving a composite ophthalmic index (including eyelid swelling, CAS, proptosis, eyelid width, diplopia and eye muscle motility) in patients with active, moderate-to-severe TED.47 The combination group showed fewer relapses at 36 weeks, and there was no difference in the rate of adverse events.47
Targeted therapies
Recently, the focus of the management of TED has shifted to novel biological therapies that target the immune cells and receptors implicated in the pathogenesis of TED. Compared with non-specific treatments, such as corticosteroids, these targeted therapies have potentially better safety profile and greater efficacy.48–53 Figure 1 shows the change in our current treatment paradigm with the advent of biological therapies.54
Figure 1: Treatment algorithm for active, moderate-to-severe thyroid eye disease
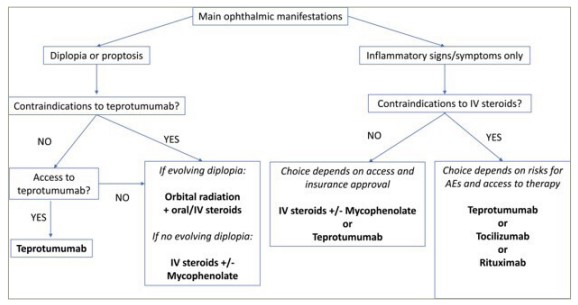
AE = adverse event; IV = intravenous.
Reproduced with permission from Kossler AL, et al., 2022 (https://creativecommons.org/licenses/by-nc-nd/4.0/).54
Rituximab
A monoclonal antibody targeting CD20, rituximab acts by depleting the body’s population of autoreactive B cells.55 Two RCTs have demonstrated mixed results in the use of rituximab for active, moderate-to-severe TED.56,57 Salvi et al. showed the superiority of rituximab (1,000 mg IV twice weekly ) compared with IV methylprednisolone in improving CAS at 24 weeks in 32 patients with eyelid retraction (13 versus 0%, respectively).56 No improvement in mean proptosis or diplopia was found in either group. Stan et al. showed no difference between rituximab (500 mg once) and placebo in 25 patients with CAS, proptosis, diplopia or eyelid retraction at 24 or 52 weeks.57 The study populations in the two studies differed in the percentage of smokers included (59 versus 16%), the average duration of TED (4 versus 12 months) and baseline CAS (≥3 versus ≥4), which may contribute to the difference in findings between the two RCTs. Notably, the rates of adverse events from rituximab therapy were high (8/13 patients and 13/15 patients) and included infusion reactions, transaminitis, hypotension, aggravation of inflammatory bowel disease and transient loss of vision.56,57
Adalimumab
Adalimumab is an anti-TNF-α agent that has been used to treat various autoimmune conditions, including active TED, with limited evidence. In a small retrospective study, 4 out of 10 patients reported subjective improvements in diplopia, pain and swelling, but no significant objective improvements on examination were measured.58 Larger and more rigorous studies are needed to establish the effectiveness of adalimumab in TED.
Infliximab
Infliximab is a monoclonal antibody that also targets TNF-α. Case reports of its use in patients with steroid-resistant, severe TED have shown improvements in visual acuity (VA) and CAS after one to three doses.59,60 Further investigation with larger studies is needed to determine the efficacy of infliximab in TED.
Tocilizumab
Tocilizumab is an IL-6 monoclonal antibody used to treat active TED. A proinflammatory cytokine, IL-6 is found in high concentrations in patients with TED.61,62 IL-6 stimulates the expression of TSHR in orbital fibroblasts, thereby potentiating the pathways implicated in disease.63 An RCT evaluated tocilizumab, administered as four infusions every 4 weeks, in 32 patients with corticosteroid-resistant, moderate-to-severe TED. Tocilizumab was found to significantly reduce CAS by two points or greater in 93% of patients at week 16 versus 59% in the placebo group.64 No improvement in diplopia was seen, and initial improvements in CAS and proptosis were lost at 40 weeks of follow-up.64 Furthermore, the recurrence rate was high, and side effects – such as hypercholesterolaemia, transaminitis, neutropenia and infections – were common, with more than one adverse event occurring in 60% of treated patients versus 24% of placebo.65
Teprotumumab
Teprotumumab, an antibody targeted against the IGF-1R, is the first FDA-approved therapy for active, moderate-to-severe TED.3 Teprotumumab reduces the production of proinflammatory cytokines by inhibiting both IGF-1R and TSHR signalling.66 The teprotumumab regimen consists of eight infusions administered every 3 weeks, with an initial dosing of 10 mg/kg for the first infusion, followed by 20 mg/kg for the remaining infusions.4 The OPTIC phase III trial (Treatment of Graves’ Orbitopathy [Thyroid Eye Disease] to Reduce Proptosis With Teprotumumab Infusions in a Randomized, Placebo-Controlled, Clinical Study; ClinicalTrials.gov identifier: NCT03298867) showed that proptosis improved in 83% of the teprotumumab group versus 10% of the placebo group, with a mean improvement of 3.32 mm.4,67 Diplopia improved by one grade or more in 68% of the treatment group versus 29% of placebo. CAS reduced to 0 or 1 in 59% of the treatment group versus 21% of placebo.4
Durability data
Pooled long-term data from two randomized, placebo-controlled, multicenter trials (Teprotumumab [RV 001] Treatment in Patients With Active Thyroid Eye Disease. ClinicalTrials.gov identifier: NCT01868997; Treatment of Graves’ Orbitopathy [Thyroid Eye Disease] to Reduce Proptosis With Teprotumumab Infusions in a Randomized, Placebo-Controlled, Clinical Study (OPTIC). ClinicalTrials.gov identifier: NCT03298867)67,68 showed proptosis response (defined as ≥2mm reduction in the study eye) in 62 of 71 patients (87%) 7 weeks after final dose of teprotumumab and 38 of 57 patients (67%) 51 weeks after. Diplopia response (≥1 Bahn-Gorman grade) was seen in 38 of 58 patients (66%) 7 weeks after and 33 of 48 patients (69%) 51 weeks after. The composite outcome (defined as improvement seen in at least 2 of the following: CAS, proptosis, lid aperture, diplopia, and/or globe motility) improved in 66 of 72 patients (92%) and 48 of 58 patients (83%) after 7 and 51 weeks, respectively.69
Adverse events
Most adverse events reported in the clinical trials were mild to moderate, including muscle spasms, nausea, diarrhoea, fatigue, hyperglycaemia and alopecia.4,70 In the teprotumumab group, 5–12% of patients withdrew early from the study due to a serious adverse event, although half of them were categorized as likely unrelated to the treatment (such as pneumothorax, urinary retention and Escherichia sepsis).4,70 However, recent studies suggest that hearing impairment may be more prevalent and serious than previously reported.71 Sears et al. reported that 81.5% (22 of 27 patients) of patients who received at least four infusions of teprotumumab complained of otologic symptoms (tinnitus, autophony, ear plugging and hearing loss), with five patients developing objective sensorineural hearing loss and two patients with a significant decline in word recognition.72 After an average of 39.2-week post-treatment follow-up, these symptoms resolved in the majority of patients. However, 45.5% of patients with new-onset or worsening hearing loss remained symptomatic.72 Until the risk factors for hearing dysfunction are better understood, the authors recommend baseline audiogram testing prior to starting teprotumumab, repeat testing during therapy if otologic symptoms develop and post-treatment audiometry. The authors also recommend ear, nose and throat team involvement and discontinuation of teprotumumab if significant hearing loss develops during therapy. Among others, additional concerning adverse events include hyperglycaemia and the development or exacerbation of pre-existing inflammatory bowel disease after initiating teprotumumab therapy, underlining the importance for clinicians to exercise caution in patient selection.73–75 Providers must weigh the risks, benefits and alternatives to therapy and screen, educate and monitor all patients during therapy.
Teprotumumab for dysthyroid optic neuropathy
Several case reports describe the efficacy of teprotumumab in treating dysthyroid optic neuropathy (DON), with improvements in relative afferent pupillary defect (RAPD), VA, Humphrey visual fields, CAS, proptosis and extraocular muscle size after two to three infusions.76–79 In a case series of 10 patients with DON, 70% of patients showed improvement in RAPD and/or VA after two infusions.80 On average, patients achieved 5.25 points of CAS improvement and 4.7 mm of proptosis reduction, and six of the seven patients with colour deficiency recovered their colour vision.80 Additional clinical studies and long-term data are needed to further explore the efficacy of teprotumumab for sight-threatening TED.
Teprotumumab for long-disease-duration thyroid eye disease
The efficacy of teprotumumab in patients with long disease duration or who may need retreatment is currently being investigated. The OPTIC X clinical trial (Treatment of Graves’ Orbitopathy to Reduce Proptosis With Teprotumumab Infusions in an Open-Label Clinical Extension Study; ClinicalTrials.gov identifier: NCT03461211) enrolled patients who were proptosis non-responders in the earlier OPTIC trial, those who received placebo in OPTIC and those who met the criteria for re-treatment due to relapse in the follow-up period (defined as a loss of at least 2 mm of their week 24 proptosis improvement or substantial increase in the number of inflammatory signs).81 OPTIC X found that 89% of the OPTIC placebo patients (who had an average of 12-month duration of TED) were proptosis responders after treatment with teprotumumab.81 Five of the eight patients who relapsed after OPTIC experienced at least 2 mm of proptosis improvement with additional treatment, and of the five OPTIC non-responders, two benefitted from an additional course of teprotumumab in the OPTIC-X study.81
Other studies have reported the use of teprotumumab in long-disease-duration TED.82–84 A retrospective study of 31 patients with TED for longer than 2 years with CAS ≤3 and without any changes in the examination showed a mean reduction in proptosis by 3 mm, improvement in CAS by 1.8 and improvement in Gorman diplopia score by 0.5.83 An analysis of pre- and post-therapy imaging revealed reductions in the volume of both extraocular muscles and fat.83 A multicentre retrospective study, involving 66 patients with refractory, moderate-to-severe TED, of varying disease durations, who had failed prior therapy with corticosteroids, orbital radiation, surgical decompression, biologics or other steroid-sparing medications, demonstrated proptosis, CAS and diplopia responses of 85.9, 93.8, and 69.1%, respectively, which were comparable with response rates seen in treatment-naive patients.85 Patients experienced a mean reduction in proptosis of 3.1 ± 2.4 mm and a mean improvement in CAS of 3.8 ± 1.6. This study found no significant difference based on the disease duration or prior medical therapy used for TED, although patients with prior decompression surgery experienced significantly lower diplopia response (46.7% versus 77.5%, p = 0.014) and proptosis response (75.0% versus 90.9%, p = 0.045) when compared with nondecompression patients.85
Medical management for non-inflammatory (inactive) thyroid eye disease
Preliminary results from a phase IV clinical trial (A Study Evaluating TEPEZZA® Treatment in Patients With Chronic [Inactive] Thyroid Eye Disease; ClinicalTrials.gov identifier: NCT04583735)86 is evaluating the use of teprotumumab in patients with long-disease-duration TED (between 2 and 10 years) and low CAS (≤1) showed an average of 2.41 mm of proptosis reduction in the treatment group versus 0.92 mm in the placebo group at week 24. The secondary endpoint of ≥2 mm of proptosis reduction was met by 62% of patients in the treatment group versus 25% in the placebo group.87 The study did not find a significant improvement in the diplopia response rate between the two groups. This study excluded patients with prior orbital radiation, decompression surgery or strabismus surgery. Long-term studies are needed to assess the treatment response durability and potential reactivation or proptosis regression after therapy. Until then, teprotumumab can be considered a medical treatment alternative to orbital decompression for proptosis improvement in patients with non-inflammatory TED.
Emerging therapies
As our understanding of the pathophysiology underlying TED advances, new molecular targets are identified (see Figure 2).88 While teprotumumab is a promising new therapy, it is limited by its adverse event profile, cost of therapy and need for access to an infusion centre. New molecular targets are under investigation that address some of the limitations to current treatment options (see Table 1).89–95
Figure 2: Molecular targets for current and emerging medical therapies88
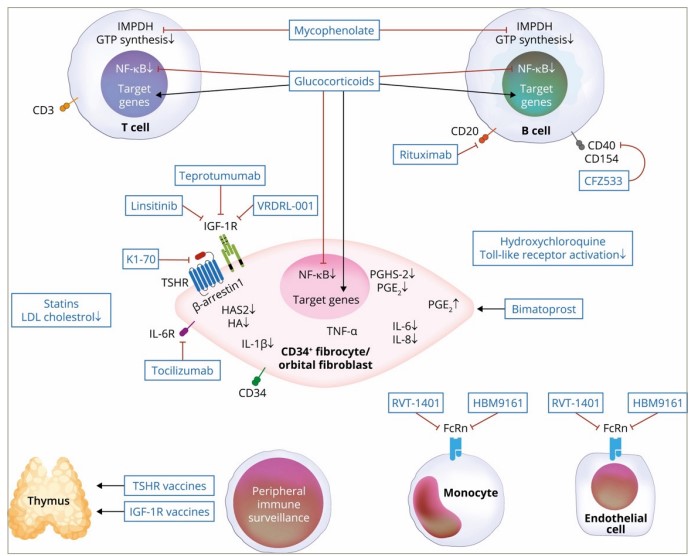
CD = cluster of differentiation; CFZ533 = anti-CD40 antagonist; FcRn = neonatal Fc receptor; GTP = guanosine-5′-triphosphate; HA = hyaluronic acid; HAS2 = hyaluronan synthase 2; HBM9161 = FcRn antagonist; IGF-1R = insulin-like growth factor-1 receptor; IL = interleukin; IMPDH = inosine 5′-monophosphate dehydrogenase; LDL = low-density lipoprotein; NF-kB = nuclear factor-κB; PGE2 = prostaglandin E2; PGHS-2 = prostaglandin-H synthase-2; RVT-1401 = FcRn antagonist; TNF-α = tumour necrosis factor-α; TSHR = thyroid stimulating hormone receptor; VRDN = a Viridian investigational drug.
Reproduced with permission from Smith TJ. 2022 (https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en).88
Table 1: Emerging therapies, their mechanisms of action, dosing, delivery and developmental stage
|
Drug name |
Mechanism of action |
Route of delivery |
Dosing |
Developmental stage |
|
VRDN-001 |
Anti-IGF-1R monoclonal antibody |
IV |
Phase 1/2: Two infusions of 3,10, or 20 mg/kg vs placebo, given 3 weeks apart Phase 3: Five invusions of 10mg/kg or placebo given 3 weeks apart. |
Phase I/II and Phase III89 |
|
VRDN-002 |
Anti-IGF-1R monoclonal antibody (extended half-life) |
SC and IV |
Single dose of 300 mg/2 mL |
Phase I90 |
|
Batoclimab |
Anti-FcRn monoclonal antibody |
SC |
2-weekly 680 mg doses followed by 4-weekly 340 mg doses |
Phase III91 |
|
Linsitinib |
Anti-IGF-1R monoclonal antibody |
Oral |
Twice a day for 6 months |
Phase IIb92 |
|
Lonigutamab |
Anti-IGF-1R monoclonal antibody |
SC |
Single dose given at day 1 and day 21, weekly dosing and monthly dosing |
Phase I/II93 |
|
K1-70 |
Anti-TSHR monoclonal antibody |
IM and IV |
Single IM injection (at 0.2, 1, 5 or 25 mg/dose) or IV infusion (at 50 or 150 mg/dose) |
Phase I94 |
|
Secukinumab |
Anti-IL-17A monoclonal antibody |
SC |
300 mg at baseline, week 1, week 2, week 3, week 4, week 8 and week 12 |
Phase II/III95 |
FcRn = neonatal Fc receptor;IGF-1R = insulin-like growth factor-1 receptor;IL = interleukin;IM = intramuscular;IV = intravenous;SC = subcutaneous;TSHR = thyroid stimulating hormone receptor;VRDN = a Viridian investigational drug.
VRDN-001, VRDN-002 and VRDN-003
VRDN-001, a monoclonal antibody against the IGF-1R, is under investigation as a treatment option for TED. The phase I/II clinical trial (A Safety, Tolerability and Efficacy Study of VRDN 001 in Healthy Volunteers and Persons With Thyroid Eye Disease [TED]; ClinicalTrials.gov identifier: NCT05176639) evaluated the efficacy of varying doses of the drug at 3 and 10 mg/kg.96 The 3 mg/kg treatment group showed a mean reduction of 1.5 mm in proptosis from baseline, 33% proptosis response rate and CAS improvement by 2.0. The 10 mg/kg group showed a mean reduction of 1.8 mm in proptosis, 50% proptosis response rate and CAS improvement of 1.8.96 None of the patients with diplopia at baseline achieved the resolution of their symptoms. There were no serious adverse events, discontinuations or infusion reactions reported.96
VRDN-002 is an anti-IGF-1R antibody compound with a modified Fc region engineered to extend the half-life of the drug. In phase I clinical trials involving healthy volunteers, VRDN-002 showed an approximately four times half-life extension when compared with VRDN-001, and patients’ measured IGF-1 serum levels were elevated when compared with placebo.90 Additionally, VRDN-003 is another half-life-extended version of VRDN-001. In preclinical studies involving monkeys, VRDN-003 was found to have a similar pharmacology to VRDN-001, with twice the half-life when delivered intravenously and subcutaneously.97 The increased half-lives of VRDN-002 and VRDN-003 may enable therapeutic levels of the drugs to be achieved with low-volume subcutaneous (SC) delivery, potentially decreasing the burden of care for patients with TED. Studies are ongoing, evaluating these potential therapeutic options.
Batoclimab
Batoclimab is a monoclonal antibody targeting the neonatal Fc receptor (FcRn) that has been proposed for the treatment of several autoimmune diseases, including TED.98 FcRn transports immunoglobulin G (IgG) and prevents their lysosomal degradation.99 Batoclimab blocks FcRn-mediated recycling of IgG and increases the catabolism of IgG in the body. In TED, this may increase the degradation of pathogenetic autoantibodies against the TSHR and IGF-1R. Administered as a weekly SC injection, batoclimab allows for the possibility of at-home treatment. Proof-of-concept, randomized, double-blind placebo-controlled trials (A Proof-of-Concept Study to Assess Batoclimab in Participants With Graves’ Disease; ClinicalTrials.gov identifier: NCT05907668) evaluated 2-weekly 680 mg SC doses of batoclimab followed by 4-weekly 340 mg SC doses of batoclimab in 77 adult patients with moderate-to-severe active TED.91 Both trials showed significant decreases in anti-TSHR antibody and total IgG serum levels with batoclimab. The batoclimab group showed a significant decrease in orbital muscle volume and an improvement in quality of life when compared with the placebo group. The study found no statistically significant difference in the proptosis response between the treatment and placebo groups; however, the trial was terminated early due to an unanticipated increase in serum cholesterol. The increase in lipids is reversed upon discontinuation of the drug.100 A phase III RCT (Study to Assess Batoclimab in Participants With Active Thyroid Eye Disease; ClinicalTrials.gov identifier: NCT05524571) is currently underway to evaluate the safety and efficacy of the treatment of TED.101
Linsitinib
Linsitinib is a small-molecule inhibitor of IGF-1R. In an experimental mouse model of TED, it was found to prevent autoimmune hyperthyroidism in the early stage of the disease and reduce the infiltration of orbital tissues by T cells and macrophages.92 The drug is currently in phase IIb clinical trials (A Phase 2b, Study of Linsitinib in Subjects With Active, Moderate to Severe Thyroid Eye Disease [LIDS]; ClinicalTrials.gov identifier: NCT05276063).102 It is administered orally twice daily for 6 months to patients with active, moderate-to-severe TED.
Lonigutamab
Lonigutamab is another anti-IGF-1R monoclonal antibody designed to have a strong binding affinity to its target, thereby potentially enabling a small-volume SC injection. The drug is currently in phase I/II clinical trials, which will randomize patients to one of the three different treatment doses (Efficacy and Safety of Lonigutamab in Subjects With Thyroid Eye Disease; ClinicalTrials.gov identifier: NCT05683496).93
K1-70
K1-70 is a human monoclonal antibody targeting TSHR. In a phase I clinical trial, 18 patients with Graves’ disease were treated with a single intramuscular (IM) injection (at 0.2, 1, 5 or 25 mg/dose) or IV infusion (at 50 or 150 mg/dose). T3, T4 and TSH levels progressed into hypothyroid ranges with an IM dose of 25 mg or an IV dose of 50 or 150 mg. There were no serious adverse events that required discontinuation, dose reduction or dose interruption of therapy. Additional studies are needed to determine the efficacy of TSHR inhibition in patients with TED.94
Secukinumab
Secukinumab is a human monoclonal anti-IL-17A antibody already approved for the treatment of psoriatic arthritis, ankylosing spondylitis and severe plaque psoriasis.103,104 IL-17 is a group of proinflammatory cytokines secreted by T cells, mast cells and neutrophils, with IL-17A being the key effector cytokine. The binding of IL-17 to its receptor activates downstream pathways involved in the amplification of other cytokines, including TNF-α, cellular hyperproliferation and T-cell infiltration, leading to an increased inflammatory response.105 By blocking IL-17A, secukinumab may potentially decrease the expression of cytokines and prevent the inflammatory processes involved in TED. Secukinumab is now currently in phase II/III clinical trials for patients with active, moderate-to-severe TED. It is delivered as an SC injection of 300 mg at baseline, week 1, week 2, week 3, week 4, week 8 and week 12 (A Study of the Efficacy and Safety of Secukinumab 300 mg in Patients With Thyroid Eye Disease; ClinicalTrials.gov identifier: NCT04737330).95
Conclusions
Recent advances in our understanding of the molecular underpinnings of TED have changed the treatment landscape. Teprotumumab, the only FDA-approved therapy for TED, has shown efficacy in improving proptosis, diplopia and inflammatory signs in patients with active moderate-to-severe disease. Questions remain regarding its cost-effectiveness, ease of access and severity of adverse events. Several emerging therapies under investigation similarly target IGF-1R, each with different binding affinities, dosing regiments and delivery approaches. Other therapies target TSHR, active cytokines in the TED inflammatory pathway or the neonatal FcRn. Investigating treatment options may bring new insights into the pathophysiology of TED and address the limitations of current treatment options, including cost, access, safety and limited efficacy in certain types of TED. Additional research identifying different phenotypes of TED, risk factors for adverse events to therapy, objective testing parameters, biomarkers for better diagnosis and measurement of response to therapy and the role of surgery for TED with or without combination with medical treatment are needed.