The importance of objective and quantitative measurement methods in uveitis – laser flare photometry
Carl P Herbort Jr
Centre for Ophthalmic Specialised Care at Clinic Montchoisi Teaching Centre, Lausanne, Switzerland; University of Lausanne, Lausanne, Switzerland
Laser flare photometry (LFP) involves measuring the level of scattered light (in photons per millisecond [ph/ms]) from a laser beam directed at the anterior chamber of the eye in order to assess the amount and size of proteins and cells in the aqueous humour, which provides an indirect measure of ocular inflammation.1 The use of LFP was the first step towards quantitative measurements of ocular inflammation, as conventional evaluation of flare using a slit-lamp has only five levels of grading (0–4+), whereas LFP grading ranges from 3–1,000 ph/ms (normal eyes typically have a value of ≤5 ph/ms),2 a very large increase in precision.1
Currently, the most significant use of LFP is in the management of uveitis. The first report of LFP use in this indication was published in 19972 and now many centres in Europe are equipped to carry out the measurement. Despite this, LFP it is not yet approved for clinical use worldwide (for example, it is not approved in the United States). Here we describe two clinical cases to highlight the use and benefits of LFP in patients with various forms of uveitis.
Case 1
A female patient, who was diagnosed with juvenile idiopathic arthritis (JIA) at the age of 4 years and uveitis at the age of 8 years, required a cataract operation involving intraocular lens (IOL) implantation in the right eye at the age of 14 years. During this procedure, inflammation occurred, resulting in irreversible cystoid macular oedema, macula destruction and a progressive loss of visual acuity (VA; final recorded value: 0.08). Subsequently, the patient presented with a decrease in VA in the left eye and cataract extraction and IOL implantation was scheduled. LFP showed that pre-operative flare levels in the right and left eyes were 228 and 207 ph/ms, respectively, showing that ocular inflammation was not adequately controlled, despite successful management of systemic JIA with methotrexate. Additional treatment with anti-tumour necrosis factor alpha (TNFa) and systemic steroids reduced this to 96 ph/ms prior to surgery.
Post-operative flare levels (44 ph/ms) indicated minimal ocular inflammation, and best-corrected VA had improved from 0.1 pre-surgery to 0.4–0.5. However, the following day, flare had risen to 491.9 ph/ms (VA=0.2) and was treated with eye drops, anti-inflammatory agents and orbital floor and posterior sub-Tenon’s injections (betamethasone and triamcinolone). Despite this, the next day flare had risen to 567.9 ph/ms. Upon further investigation this was attributed to agglomerated protein from fibrin moving into solution. Treatment with a second orbital floor injection of betamethasone led to a gradual decrease of flare and improvement in VA over the next few days (at day 8: VA=1.0; flare=185 ph/ms).
This level of post-operative control was only achieved due to the precision of LFP, which enabled the ophthalmologist to immediately react to the changing situation and adapt therapeutic interventions as required.
LFP can be used to monitor intraocular inflammation, not only in anterior uveitis, but also in posterior forms of uveitis such as necrotic herpetic retinopathies, including acute retinal necrosis or bilateral acute retinal necrosis.
Case 2
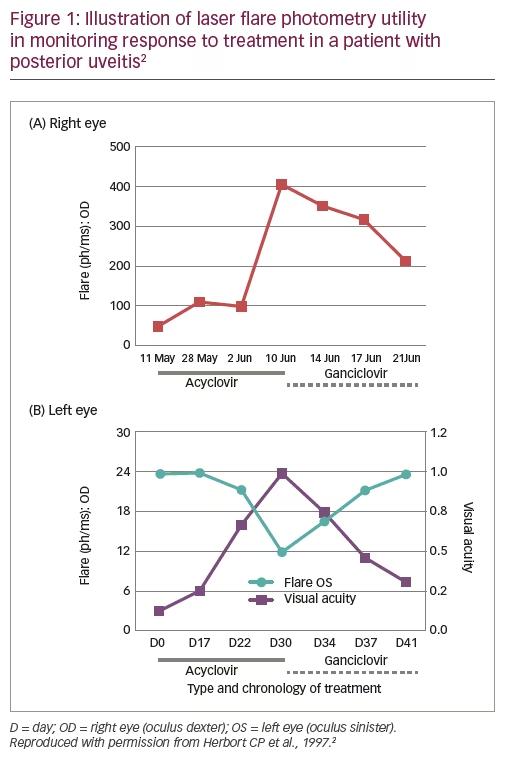
A 71-year-old male patient who had received surgery for colon cancer, as well as pre- and post-operative radiotherapy, presented 1 year later with decreased VA (0.05) in the right eye, typical granulomatous anterior uveitis with mutton-fat deposits, numerous whitish lesions of the posterior pole and an extensive zone of retinal necrosis.2 He was diagnosed with bilateral acute retinal necrosis and treatment was initiated with high dose acyclovir (10 mg/kg three times daily). LFP monitoring showed that although flare initially decreased, it then increased over the next 1.5 weeks, not only in the right eye (from ~100 ph/ms to ~400 ph/ms) but also sub-clinically in the left eye (Figure 1).2 These changes could be detected several days before VA was affected. Treatment was therefore switched to ganciclovir (5 mg/kg twice daily) and both flare and VA improved over the next 10 days (Figure 1).2
By providing a precise measure of intraocular inflammation at any given time, LFP can be used to assess treatment efficacy and enable early changes in therapeutic interventions.
In conclusion, LFP represents substantial technological progress for uveitis, transforming flare from a qualitative to a quantitative measure of intraocular inflammatory activity. As such, it can be considered one of the major recent developments in the treatment of intraocular inflammatory disorders. It is therefore essential for centres specialising in intraocular inflammation or involved in ophthalmologic clinical studies on intraocular inflammation to perform LFP monitoring. q
Anterior chamber flare as a surrogate marker of retinal vascular leakage
Ilknur Tugal-Tutkun
Department of Ophthalmology, Istanbul University, Istanbul, Turkey
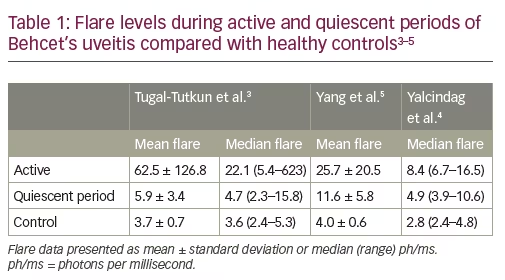
Laser flare photometry provides a quantitative measure of intraocular inflammation which correlates with both anterior and posterior segment inflammation during uveitis exacerbations.1,2 Three studies of LFP in patients with Behcet’s uveitis have also shown evidence of subclinical elevated flare during periods of quiescence between exacerbations (Table 1).3–5 In the 2008 study by Tugal-Tutkun et al., LFP flare count was shown to correlate significantly with fluorescein angiographic (FA) leakage (determined using a seven-point score [ranging from 0–7] representing leaks at the optic disc, macula and four quadrants; p=0.01) in eyes with no sign of active inflammation; there was an increased risk of recurrent uveitis in eyes with a flare count of >6 ph/ms.3 Subsequently, a more sophisticated scoring system for posterior segment inflammation was developed and validated by the Angiography Scoring for Uveitis Working Group (ASUWOG), which incorporates both fluorescein and indocyanine green angiography.6,7 Supporting earlier findings, a 2017 study by Yalcindag et al. (n=45; 78 eyes, 20 with no inflammation) demonstrated that LFP flare levels had a significant correlation with ASUWOG FA score (correlation coefficient: 0.368; p=0.001).4
Preliminary results from a recent study at the University of Istanbul in patients with Behcet’s uveitis also showed (i) that flare measured by LFP was significantly correlated with ASUWOG FA score and central macular thickness, and (ii) in clinically inactive eyes with fluorescein leakage, flare levels were elevated compared with eyes with no fluorescein leakage. Preliminary results from a separate study of 15 patients with idiopathic intermediate uveitis at the same department also showed a significant correlation between flare and ASUWOG FA score, but not between flare and central macular thickness (Guner MK, et al. 2017; unpublished data).
In conclusion, study evidence suggests that sub-clinically elevated flare may indicate FA leakage during periods of quiescence in patients with Behcet’s uveitis and that anterior chamber flare measured by LFP may also be a surrogate marker of FA leakage in idiopathic uveitis. In addition, as a quick and non-invasive procedure, LFP may reduce the need for FA monitoring of persistent retinal vascular inflammation in Behcet’s uveitis. q
Previous studies carried out to determine how clinical assessments of anterior chamber inflammation correlate with the quantitative measurements of LFP indicate that clinical grading is not an exact science and that LFP provides an accurate, reproducible, non-invasive assessment of aqueous flare that is not affected by pupillary dilation.8,9
The objective of our study was to establish the proportion of cases where LFP led to the modification of an initial therapeutic decision (which was based on clinical assessment) and to assess correlations for anterior chamber flare grading between clinicians with different levels of experience.10 Consecutive patients attending the uveitis clinic at the Moorfields Eye Hospital, London, UK were included in the study. A clinical assessment of flare was performed separately by a senior and a junior ophthalmologist (both of whom were blinded to each other’s assessments) and therapeutic decisions made accordingly. Flare readings using LFP were then recorded by a third ophthalmologist (again blinded to the initial clinical assessments) and presented to the senior ophthalmologist. The initial therapeutic decisions were then reviewed, the patients informed and follow-up assessments (both clinical and LFP) carried out after 4 weeks.
In total, 36 patients (59 eyes) were enrolled in the study; 20 (56%) were female and the mean (± standard deviation) patient age was aged 50.9 ± 16.6 years.10 For the initial clinical assessments of aqueous flare grade, there was exact agreement between both ophthalmologists for 30 eyes (51%), one-step disagreement in 26 eyes (44%) and more than one-step disagreement in three eyes (5%).10 Subsequent LFP readings led to a change of treatment management in eight eyes (14%). While there was concordance between senior clinician grading and LFP readings, with little overlap of LFP ranges across clinical grades, there was substantial overlap of LFP ranges across grades assessed by the less experienced clinician (Figure 2).
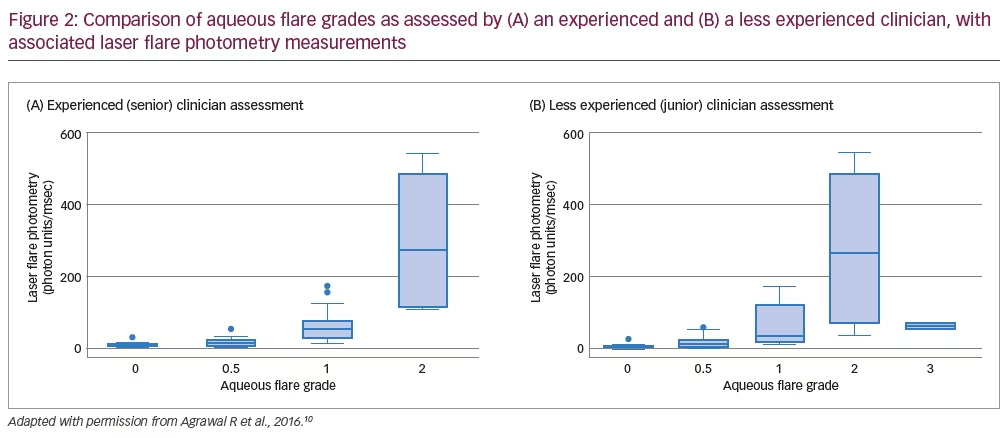
At the 4-week follow-up assessment, 34 eyes (58%) were assessed using LFP. Among these patients, there was exact agreement between ophthalmologists on the clinical grade of aqueous flare for 22 eyes (65%), one-step disagreement in 11 eyes (32%) and more than one-step disagreement in one eye (3%).10 Overall, similar improvements in the mean clinical grade of aqueous flare (senior assessment) and the median LFP were observed at follow up. In five eyes (15%), LFP readings led to a change in treatment management, which consisted of an increase in the frequency of topical steroids (three eyes) or a tapering of topical steroid usage (two eyes).
In conclusion, of all the assessments made by both clinicians, 11% of treatment decisions based on clinical flare assessments were changed once LFP measurements were obtained, with follow-up data confirming the benefit of the modified interventions. LFP can therefore be a valuable tool to correctly ascertain the grading of aqueous flare in patients with uveitis, particularly for less-experienced clinicians. q
ADJUVITE – A double-blinded, randomised, placebo-controlled trial of adalimumab in early onset, chronic, juvenile idiopathic arthritis-associated uveitis
Bahram Bodaghi
Department of Ophthalmology, University Hospital Pitie-Salpetriere, Paris, France
Juvenile idiopathic arthritis (JIA)-associated uveitis is one of the most challenging inflammatory conditions in the paediatric population. Despite this, evidence-based data on therapeutic options is limited, though data from the National Italian Registry (up to December 2009) has previously indicated that adalimumab and infliximab may provide some therapeutic benefits.11
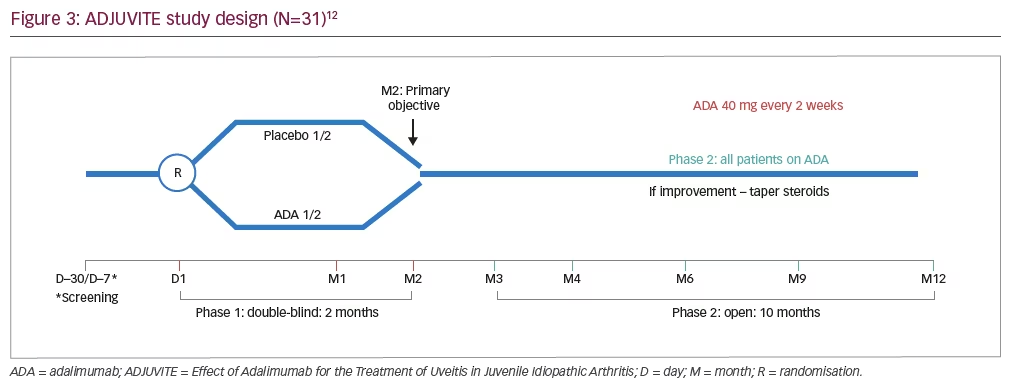
ADJUVITE (Effect of Adalimumab for the Treatment of Uveitis in Juvenile Idiopathic Arthritis) was a 12-month, investigator-initiated, phase III, multicentre, double-blind, placebo-controlled trial of adalimumab in patients with JIA-associated uveitis (ClinicalTrials.gov Identifier: NCT01385826; Figure 3).12 Inclusion criteria were: a diagnosis of active JIA-associated uveitis; age of >4 years; weight of >15 kg; LFP value of >30 ph/ms; a resistance or intolerance to topical corticosteroids; and a resistance to methotrexate.12 Patients were excluded from the study if they had: systemic JIA; previous treatment with anti-TNF agents; a contraindication to systemic immunosuppressants or biologics; or other ocular contraindications (including chronic rupture of the blood–aqueous barrier, uncontrolled glaucoma, any other major ocular complication or an inability to monitor flare using LFP).12 Eligible patients were randomised to receive subcutaneous adalimumab (40 mg for children aged 13 years or over, 24 mg/m2 [body surface area] for children younger than 13 years [up to a maximum of 40 mg] every 2 weeks) or placebo.
The primary study endpoint was the proportion of patients showing a treatment response at 2 months (defined as a flare reduction from baseline of 30% as measured by LFP, with no worsening of anterior chamber inflammation based on the Standardisation of Uveitis Nomenclature [SUN] criteria).12–14 Key secondary endpoints included the incidence of adverse events at 2 and 12 months, the proportion of eyes with active uveitis (as assessed by SUN criteria) up to 12 months, and the reduction in corticosteroid use from 2 to 12 months.12
In total, 31 patients were enrolled in the study and were included in the intent-to-treat population; 28 (90%) were female, 24 (78%) had bilateral uveitis and the mean age and duration of uveitis were similar between treatment groups.12 At 2 months, there was a significantly greater proportion of responders in the adalimumab group compared with the placebo group (56% [9/16] versus 20% [3/15]; p=0.038; 95% confidence interval: 0.94–8.45]).12 During the study, six serious adverse events were reported in five patients (worsening of ocular hypertony [n=2], hepatic cytolysis [n=2], tonsillitis [n=1] and worsening of cataract [n=1]), none of which were considered related to the study treatment by the investigator.12 Overall, 29 (94%) patients completed 12 months of treatment with adalimumab and two (6%) withdrew due to adverse events; one withdrew from the study at day 14 (from the adalimumab group) due to ocular hypertony and worsening of uveitis, and one withdrew during the open-label phase at month 9 due to uveitis relapse and worsening of rheumatic pain.12
These findings are consistent with the results from the SYCAMORE study (which did not use LFP), a multicentre, double-blind, randomised, placebo-controlled trial of adalimumab (plus methotrexate) in children or adolescents aged ≥2 years with JIA-associated uveitis.15 Overall, treatment failure (defined according to a multicomponent intraocular inflammation score that was based on SUN criteria) occurred in significantly fewer patients in the adalimumab treatment group compared with the placebo group (16 [27%] versus 18 [60%]; p<0.0001).15 However, more adverse events and serious adverse events occurred in the adalimumab group compared with the placebo group (10.07 and 6.51 events per patient-year compared with 0.29 and 0.19 events per patient-year, respectively).15 Analysis of time to treatment failure showed separation between the adalimumab and placebo groups before week 10.15 Separation in the ADJUVITE study on the primary outcome was also observed at week 8, despite a smaller patient population, which likely reflects the sensitivity of LFP assessments.
In conclusion, the results of the ADJUVITE study showed that adalimumab significantly increased the proportion of patients with JIA uveitis (resistant to methotrexate) achieving a treatment response. This was the first clinical trial to use LFP values as a primary outcome and the results highlight the utility of measuring anterior chamber flare using LFP, particularly in JIA uveitis. q
Correlation of inflammatory cytokines in the aqueous humour with aqueous flare in patients with proliferative diabetic retinopathy
Masaru Takeuchi
Department of Ophthalmology, National Defense Medical College, Saitama, Japan
Diabetic retinopathy (DR) is one of the most serious complications of diabetes, with sight-threatening DR occurring in about 8% of patients with diabetes over the age of 40 years.16 Results from a recent study have shown that inflammatory processes are involved in the pathogenesis of DR, with increased levels of inflammation mediators, such as interleukin (IL)-1β, IL-6, IL-8, and TNFa, in the vitreous fluids of patients with proliferative diabetic retinopathy (PDR). CD4+ T-helper (Th) cells are also thought to infiltrate the vitreous.17 Furthermore, several studies have shown that aqueous flare is increased in patients with DR and that the intensity of this flare is correlated with the different stages and severities of DR.18–20
The aim of the study presented here was to identify inflammatory cytokines in the aqueous humour associated with aqueous flare in patients with PDR. In total, eight patients with PDR and 15 patients with cataracts (no PDR) were included in the study (mean age: 55.5 and 72.4 years, respectively). Aqueous flare was measured using LFP and pre-cataract surgery levels of IL-2, IL-4, IL-6, IL-8, IL-10 and TNFα in the aqueous humour were measured using a beads-array system.
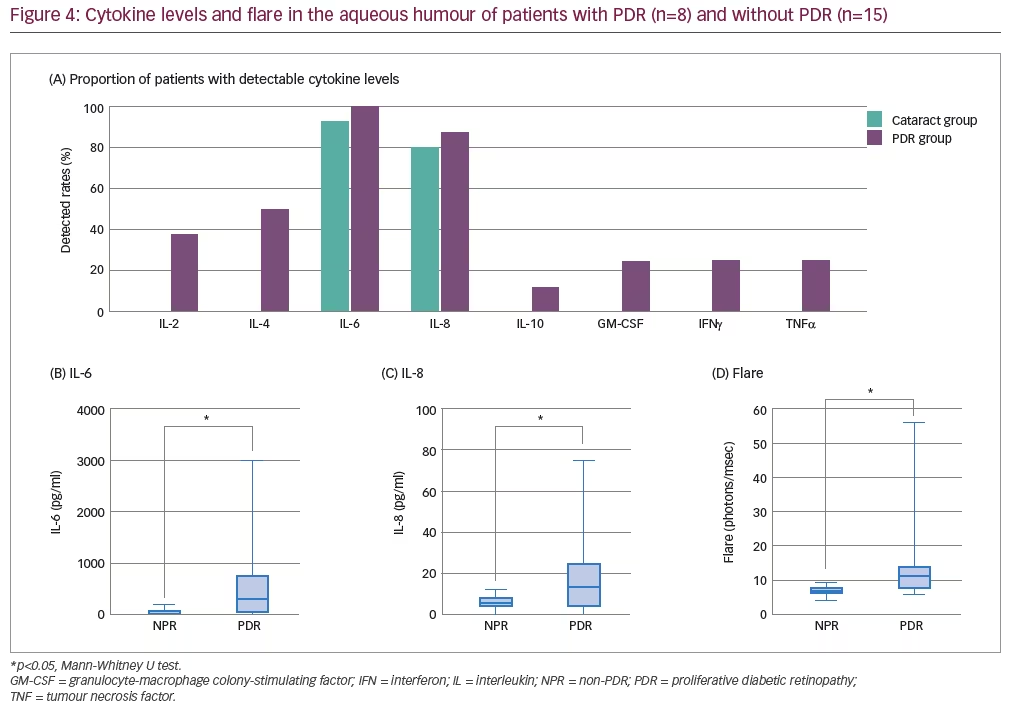
Overall, IL-2, IL-4, IL-6, IL-8, IL-10 and TNFα were detected in the aqueous humour of more patients with PDR than without PDR, although IL-6 and IL-8 were detected in >80% of eyes in both groups (Figure 4). Aqueous flare and the levels of IL-6 and IL-8 in the aqueous humour were found to be significantly greater in patients with PDR versus patients with no PDR (Figure 4; p<0.05). In addition, IL-6 levels were found to be significantly greater than IL-8 levels in patients with PDR (p<0.05). Aqueous flare itself was significantly correlated with IL-6 levels in both the aqueous humour and the vitreous of patients with PDR (R2: 0.831 and 0.599, respectively; both p<0.05), but only with IL-8 in the aqueous humour (R2: 0.743; p<0.05). While vascular endothelial growth factor (VEGF) was not measured in this study, Funatsu et al. have previously reported a significant positive relationship between VEGF and IL-6 levels in both the aqueous humour and vitreous of 26 patients with PDR.21
In conclusion, aqueous flare measured by LFP, which enables a quantitative assessment of blood-aqueous barrier impairment, reflects the progression of DR and is associated with increased aqueous humour and vitreous levels of IL-6 (and potentially VEGF). q
Laser flare photometry a prominent factor among predictors of recurrences in Vogt-Koyanagi-Harada disease
Kazuichi Maruyama
Department of Innovative Visual Science, Osaka University Graduate School of Medicine, Osaka, Japan
Vogt-Koyanagi-Harada (VKH) disease is a T-cell-mediated autoimmune disease directed against melanocytes. Symptoms include uveitis, skin depigmentation, sensory deafness or meningitis.22 While the prognosis of VKH is usually positive with appropriate steroid treatment, recurrences can occur if diagnosis is delayed or treatment is inadequate (e.g. rapid tapering of steroid therapy).23–26 Even with appropriate diagnostic and treatment strategies, approximately 20–25% of VKH cases will recur.24 When VKH does recur, it usually presents with features of chronic anterior uveitis (though posterior segment involvement can also occur), inflammation is often harder to control and the prognosis worsens.23,27
The objective of the study presented here was to identify predictors of VKH recurrence in patients admitted to Tohoku University Hospital, Japan, who were diagnosed with VKH from October 2012 to May 2016.23 Patients underwent examinations at baseline (admittance) and on days 4, 14, 30 and 60. Assessments included anterior segment optical coherence tomography (OCT), LFP, ultrasound biomicroscopy, angiography, laser speckle flowgraphy and deep-range imaging OCT.23 Following the initial diagnosis of VKH, patients were treated with a high steroid dose (e.g. prednisolone 60 mg/day), which was then tapered. Patients were monitored in the hospital until the steroid dose had been reduced to 20–30 mg/day, then via outpatient visits.23 VKH recurrence was defined (using SUN inflammation criteria) as the occurrence of anterior chamber inflammation or recurrent (two or more) retinal detachment.23
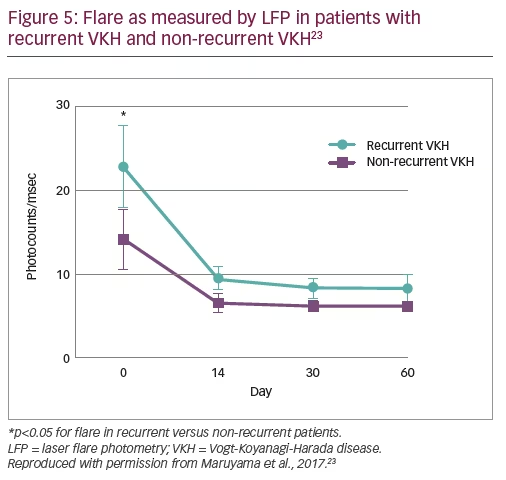
In total, 41 patients (41 eyes) were included in the study; 25 were female (61%) and the mean age was 47.5 years.23 Recurrent VKH was observed in 22 (53.7%) patients, considerably more than expected. To identify potential predictors of recurrence, analyses of patients with recurrent versus non-recurrent VKH showed no significant differences in age, time from diagnosis to treatment or the total amount of steroid administered, and no significant difference in choroidal thickness before or after treatment.23 However, significantly lower VA in the recurrent VKH group was observed prior to treatment compared with the non-recurrent group (0.27 versus 0.6; p<0.0001).23 Following treatment, VA improved in both groups and no significant differences in VA between groups was observed at day 60.23 In addition, significantly higher anterior segment flare was observed in the recurrent VKH group versus the non-recurrent VKH group prior to treatment (24.68 versus 9.04 ph/ms; p<0.05). Again, following treatment, flare was reduced in both groups and no significant difference was observed by day 60 (Figure 5).23 The mean blur rate (MBR) in the choroidal vessel area improved in both groups following treatment, but more so in the non-recurrent VKH group. As such, the recurrent VKH group had significantly lower MBR at days 14, 30 and 60.23 Following recurrence, patients received combined steroid and immunosuppressant therapy (cyclosporine or methotrexate) and 4 out of 20 (20%) experienced subsequent recurrence during follow up.23
In conclusion, results from this study show that initial VA and flare count (as measured by LFP) are predictors of VKH recurrence and that MBR response to treatment at 14, 30 and 60 days can be considered a biomarker for recurrent VKH.