Age-related macular degeneration (AMD) is the leading cause of central visual loss and legal blindness in patients over the age of 65 years.1,2 The exudative or neovascular form of AMD accounts for over 90 % of the cases with severe visual loss.3
AMD is a highly complex disease with demographic, environmental and genetic risk factors. The pathogenesis of AMD lesions remains largely unclear and current evidence suggests that AMD results from genetic predisposition and a combination of metabolic and inflammatory insult to photoreceptors and retinal pigment epithelium (RPE).4
The Seven Year Update of Macular Degeneration Patients (SEVEN-UP) study helped elucidate the challenges of long-term management of neovascular AMD (nvAMD), in particular that it is a chronic disease and that the patients remain at risk of vision loss many years after the treatment.5
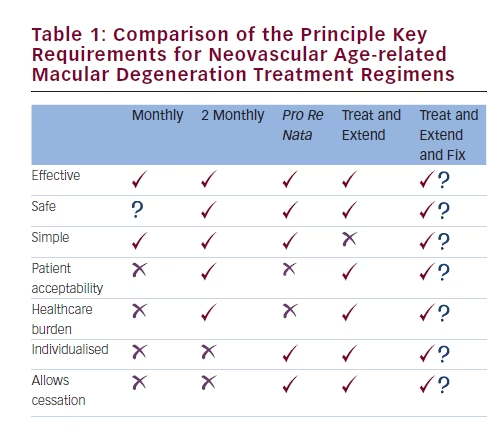
There is a need to find the best treatment regime for nvAMD that optimises visual outcomes and safety while minimising burden to patients, carers and healthcare providers (see Table 1). This editorial focuses on the pros and cons of current nvAMD treatment regimens and proposes a regimen appropriate for 2016.
Studies directly comparing treatment regimens are often complicated by the usage of different drugs, a retrospective nature, single-arm design or cross-study comparison, so interpretation can be difficult.
Currently Available Regimens for Neovascular Age-related Macular Degeneration
Regimens can be divided into an initiation and a stability/ maintenance phase.
Many studies used three initiation injections, on a monthly basis, to commence treatment.6,7 This remains a pragmatic way of commencing therapy as the majority of patients in a one dose plus re-treatmentas- necessary regimen still required three doses to achieve a similar good outcome.8
Once maximum control of activity and visual gain is achieved, ongoing disease suppression is required. The different approaches for managing this stability/maintenance phase can be considered as either ‘reactive’ or ‘proactive’ regimens. In the reactive form, treatment is given on a pro re nata
(prn) basis whenever there are signs of activity and can be either ‘tolerant’ of a degree of disease activity or ‘intolerant’ of any signs activity. Disease activity is judged by taking a composite view of new symptoms, reduced measured visual acuity, dynamic evaluation with fluorescein angiography and most important morphology on optical coherence tomography (OCT). Intraretinal fluid and cysts, subretinal fluid and sometimes change in pigment epithelial detachment height are also considered.
In the proactive form, treatment is given in a continuous manner, ideally before disease activity manifests. Proactive regimens can be further subdivided into ‘fixed’ or ‘variable’ form.
Reactive Forms
Pro Re Nata– or As-needed-basis Treatment
The Comparison of AMD Treatment Trials (CATT) treated patients with bevacizumab or ranibizumab according to either a monthly fixed dosing or an intolerant prn regimen.8 At 1 year, there was no clinically significant difference in visual acuity outcomes between the ranibizumab monthly dosing and as-needed dosing arms.
The safety and efficacy of a flexible dosing regimen of ranibizumab in an nvAMD study (Safety Assessment of Intravitreous Lucentis for AMD [SAILOR] study) had re-treatment criteria that tolerated a degree of disease asctivity.7 Visual acuity outcomes were inferior to those observed in the intolerant CATT-style regimen.
Although the intolerant as-needed regimes show favourable outcomes, it does not eliminate the burden of monthly follow-up visits.
Proactive Forms
Fixed Regimes
The landmark ANCHOR (Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD)9 and MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular AMD)10 studies used fixed monthly ranibizumab, and along with the fixed monthly aflibercept arm of the VIEW (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD) 1 and 2 studies,11 excellent visual acuity outcomes were demonstrated. Concerns about overinjection leading to a higher rate or endophthalmitis or as-of-yet-to-be-confirmed progression of atrophy steers clinicians away from this approach.12,13 Likewise, the significant burden to patients, carers and healthcare professionals make this approach impractical for many. The 2-monthly fixed-dosing arm (after three initiation doses in the first year) reduced this injection and visit burden considerably. Injection every 3 months with ranibizumab following monthly initiation however leads to significantly worse visual outcomes.14
Variable Regimes A treat-and-extend (T&E) strategy aims to individualise the needs of an eye following initiation of a treatment. The patient receives an injection at each visit with the results of the assessment of disease activity determining the interval to the next visit. If inactive, the interval is extended by 1 or 2 weeks; if active, the interval is decreased.
While there no large prospective trials comparing T&E with other dosing regimens have yet been reported, the Lucentis Compared to Avastin Study (LUCAS) trial has shown promising results in its first year.15 The improvement in visual acuity in both treatment groups, ranibizumab or bevacizumab, using a T&E protocol was comparable with visual acuity results in the monthly treatment groups of the CATT, while reducing patient visits. Open-label design studies have suggested an impact on reducing overall cost.16 It may be feared that this type of regime is complex to manage in a high-volume service.
Is There a Perfect Regimen for Neovascular Age-related Macular Degeneration in 2016?
Unfortunately, there is no single perfect regime for all nvAMD patients and a regime may need to be selected to best suit an individual depending upon disease aggression and impact on lifestyle and healthcare systems.
Available evidence suggests that individualised treatment plans provide a reasonable alternative to the monthly injection protocols. Among these, the T&E protocol seems to be increasingly favoured.
A proposed pragmatic regime to treat patients in 2016 is to initiate with three injections on a monthly basis and then find the appropriate re-treatment interval by using a T&E-style pathway. Once an interval in recognised then fix at an interval during which there is disease control for 6–12 months before reviewing the need to modify it. It is necessary to be alert to any significant increase in activity that could occur during this period. The new UK MATE study (treating nvAMD with aflibercept: a pilot, 24-month, multicentre randomised controlled trial comparing standard of care with an individualised T&E regimen) using this type of regime will inform us in 2017 to 2019.
Conclusion
In the last decade, there have been numerous advances and breakthroughs in the management of nvAMD. Despite years of trials, the optimal treatment strategy has not yet been defined and treatment decisions should be based on an in-depth discussion between the patient and physician.







