Diabetic macular oedema (DMO) is one of the leading causes of visual impairment in working adults.1 The implications of blinding due to DMO, including the loss of productivity and reduced quality of life (QoL), lead to a considerable socioeconomic burden on communities.2–5 Intravitreal treatment options, particularly with anti-vascular endothelial growth factor (anti-VEGF) agents, have shown the potential to reduce visual loss far beyond that achieved with laser therapy alone.6–10 Applying principles learnt from key clinical trials to the real world setting requires an understanding of the many patient-related factors that determine when and how treatment is started and continued. It is important for clinicians to not only understand such factors but also the evidence base for such.
The aim of this article is to bring clarity to the available data on the key patient-related factors that influence the treatment of DMO, by reviewing the literature, forming consensus statements and providing recommendations. Discussions are based around the optimum scenario in an ideal world where certain barriers were removed, for example, costing, reimbursement and resource capacity. Due to the broad nature of this topic and because anti-VEGF therapy is the commonest intravitreal intervention for DMO, the scope of the discussion is largely limited to treatment with anti-VEGF therapy.
Methods
A panel of 15 retinal experts (see Table 1) met on 14–15 November 2014 in Zurich, Switzerland, where the need for clarity of the factors that influence DMO management was initially discussed. The panel met again on 10–11 April 2015 in Berlin, Germany, and on 6–7 November 2015 in Paris, France, to take the work forward. Based on a Delphi-style methodology,11 a range of factors in the management of a patient with DMO were identified and the evidence for, and relative importance of
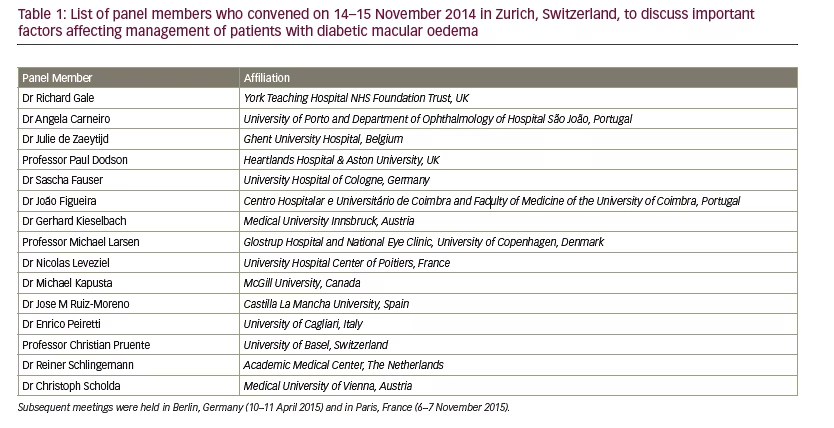
each, was discussed. A literature search complemented this process. PubMed/Medline and EMBASE searches from 1 January 2005 to 22 June 2016 were carried out using these factors as exploded mesh terms that occurred in the publication title (i.e., the term itself and a series of similar terms and subtopic terms within a particular category as used to classify each record by the database providers). Animal studies and non-English language papers were excluded. Owing to the paucity of material identified through the searches on occupation/profession, co-morbidities, compliance and family history, it was decided to broaden the searches to papers where the specified factor was not used as a major term. After removing duplicates, the search results were screened for relevance by a single retinal expert (Richard Gale). Recommendations were formed and agreed by the panel.
Results
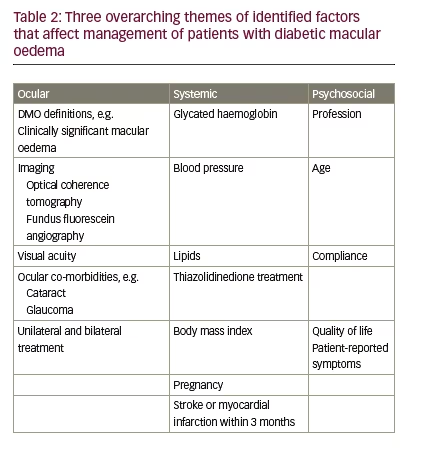
Factors were categorised into three overarching themes: ocular-specific, systemic influences and psychosocial factors (see Tables 2 and 3). Database searching identified 547 records and a further 140 records were provided from expert opinion (687 in total). Removing duplicates left 665 results, of which 606 were excluded by expert screening. In total, therefore, 59 full text articles were accessed and relevant articles discussed.
Ocular-specific factors that influence the management of diabetic macular oedema with anti-vascular endothelial growth factor therapy
(Recommendation 1)
Many overlapping definitions of DMO are currently used, for example, clinically significant macular oedema (CSMO), centre involving vision affecting (CIVA) oedema and focal or diffuse oedema.12,13 Using the correct definition is central to aligning treatment with the key clinical trials.
In 1985, the Early Treatment Diabetic Retinopathy Study (ETDRS) group defined CSMO,12 based on clinical examination alone: presence of any retinal thickening at or within 500 μm of the foveal centre, lipid exudates at or within 500 μm of the foveal centre with adjacent thickening or an area of retinal thickening at one or more Macular Photocoagulation
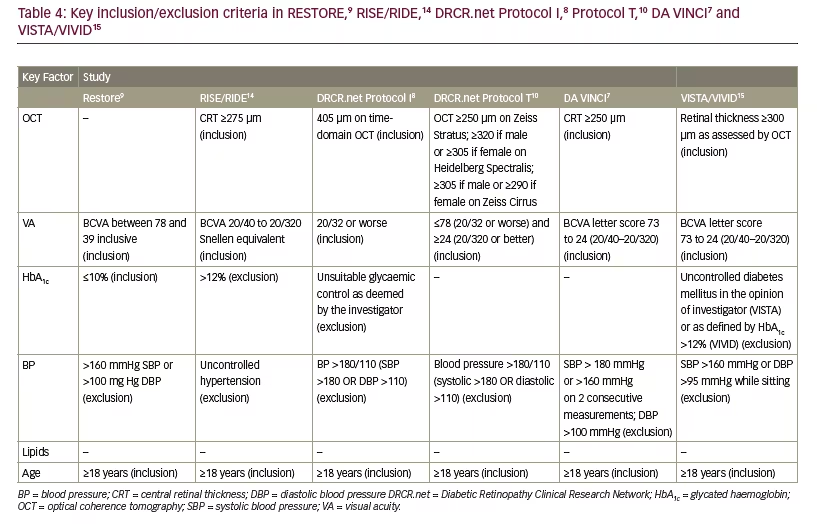
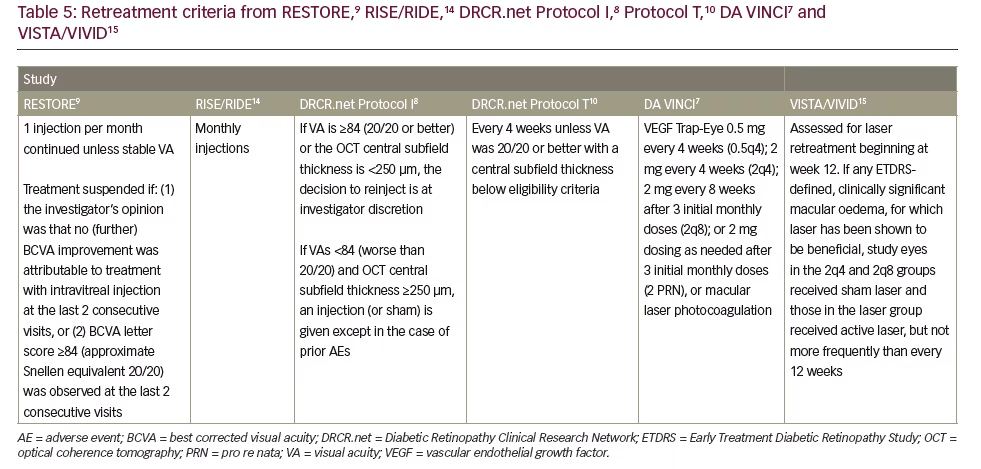
Study DA (1 disk area ≅ 1.767 mm2) within one disk diameter (1.5 mm) of the foveal centre. This definition remains useful in patients undergoing macular laser therapy; however, it is not the driver when anti-VEGF therapy is used. The inclusion criteria for the majority of the pivotal trials uses a form of ‘centre involving, vision affecting’ criteria to initiate treatment. Tables 4 and 5 demonstrate that inclusion criteria for the RISE /RIDE, Protocol I and T, DA VINCI and VISTA/VIVID studies required a combination of vision loss and documented retinal thickening.6,7,10,12,14,15 It is worth noting, however, the inclusion criteria for the RESTORE study did not require a specific CRT measurement but a clinical diagnosis of diffuse or focal thickening.9 Focal DMO was defined as more than 67% of leakage originated from leaking microaneurysms (MAs) in the whole
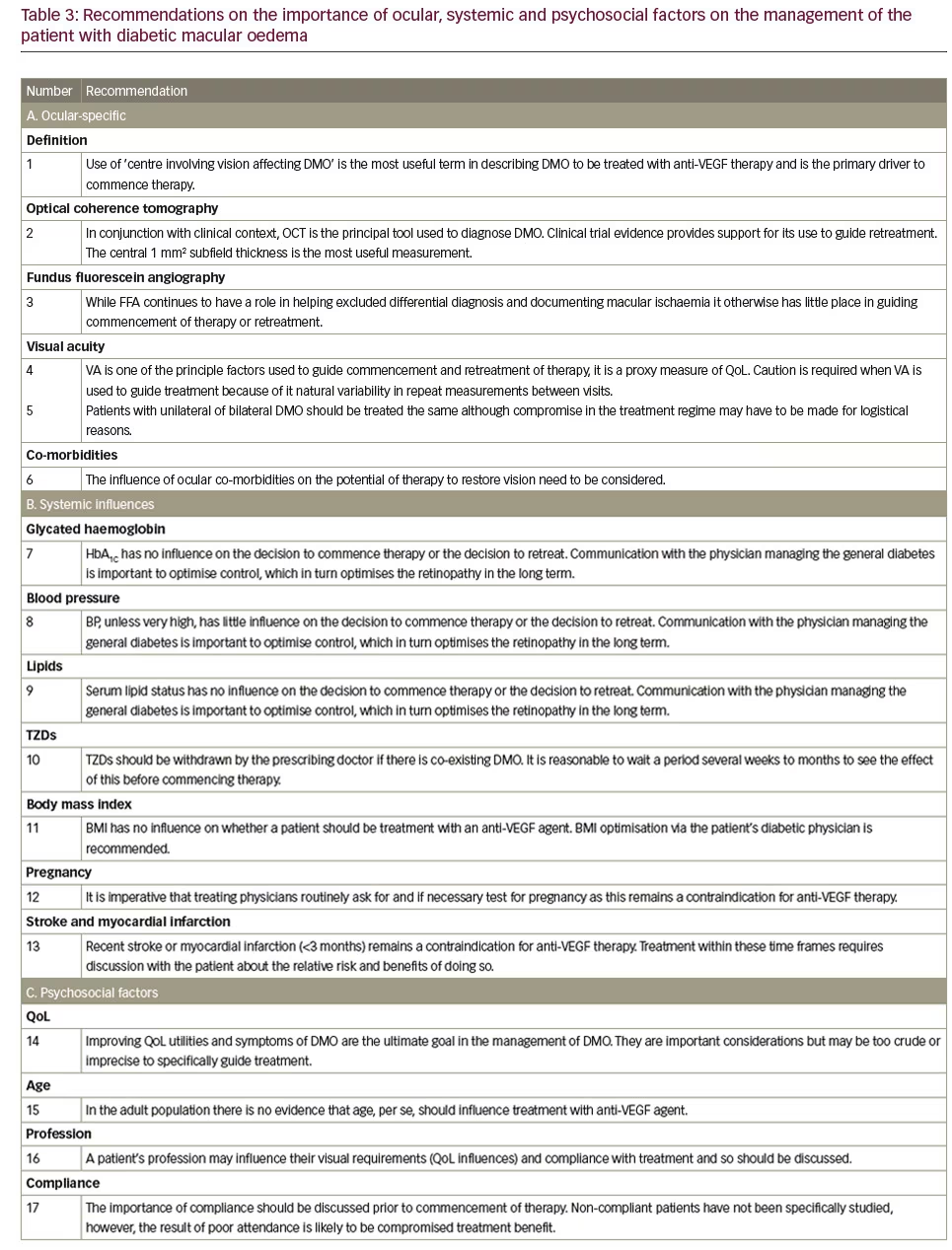
oedema area or 30–67% leakage from MAs in the whole oedema area, but >67% of the leakage originated from MAs in the central subfield.9 Diffuse DMO was defined as <33% of leakage from MAs, the rest from diffuse leaking capillaries in the whole oedema area or 30–67% leakage from MAs, but <33% of the leakage originated from MAs in the central subfield. This definition is somewhat convoluted and is not clinically important as a sub-analysis of the RESTORE data demonstrated neither group performed better than the other.9
Optical coherence tomography findings
(Recommendation 2)
Optical coherence tomography (OCT) is the most widely used tool to diagnose and manage DMO. The central 1 mm subfield is the most useful parameter in determining initiation and retreatment. Patients were recruited into the DRCR.net Protocol I who had DMO involving the fovea that, amongst other criteria, required a mean OCT central subfield thickness on OCT >250 μm on time-domain OCT.16 Inclusion for participation in DRCR.net Protocol T included: central subfield thickness on OCT ≥250 μm on Zeiss Stratus; ≥320 if male or ≥305 if female on Heidelberg Spectralis; ≥305 if male or ≥290 if female on Zeiss Cirrus and definite retinal thickening on clinical exam due to DMO involving the centre of the macula.6 In the RISE and RIDE trials, eligible participants required macular oedema with a central subfield thickness ≥275 μm on time-domain OCT.14 In the DA VINCI study, clinically significant DMO with centre involvement of the fovea was defined as a central subfield measurement of ≥250 μm on time-domain OCT.7 Central DMO involvement was defined as retinal thickening involving 1 mm central (OCT) subfield thickness (CST) in the VISTA and VIVID trials.15
Structural OCT does not provide dynamic information about macular perfusion. OCT angiography (OCT-A) is a developing technology and, as yet, its role in the routine assessment of the DMO patient is still to be fully defined OCT. Used in parallel with spectral domain (SD OCT), OCT-A presents the opportunity to learn more about the disease.17,18
Fundus fluorescein angiography findings(Recommendation 3)
Fundus fluorescein angiography (FFA) is no longer commonly used to make the diagnosis of DMO. On occasions, FFA is important to differentiate DMO from other vascular disorders, such as choroidal neovascularisation, retinal vein occlusion or macular teleangectasia. There is little direct evidence that FFA has a role in the management of DMO with anti-VEGF therapy. The key clinical trials did not specify FFA features as a part of inclusion criteria for treatment nor for retreatment (RESTORE, RISE and RIDE, Protocol I and T, DA VINCI). FFA is useful in assessing the degree of enlargement and irregularity of the foveal avascular zone, i.e., macular ischaemia. However, patients with macular ischaemia were studied in a subgroup analysis in the RESTORE study and no difference was found in their outcome – implying patients with or without macular ischaemia should be treated similarly.9 A caveat is that patients were excluded from these studies if there was a reason why vision may not improve and so patients with very severe macular ischaemia may not have been enrolled.
Visual acuity
(Recommendation 4)
Visual acuity (VA) (ETDRS score) is a key factor influencing commencement of therapy and retreatment (see Tables 4 and 5). Indeed VA assessment was used as an inclusion criteria in RESTORE,9 DRCR.net Protocols I8 and T,10 DA VINCI7 and VISTA and VIVID studies15 and furthermore to help guide retreatment (see Tables 4 and 5).8–10,15 The key clinical trials studying the use of anti-VEGF therapy in DMO only included patients with reduced vision so currently there is no evidence that supports the benefit of anti-VEGF therapy in those with unaffected, very good or very poor VA.
The DRCR.net published findings of a refracted ETDRS coefficient of repeatability of 5–13 letters between visits (the worse the VA the higher the coefficient). Caution is therefore suggested when change in VA is used prospectively as a determination of a true measure of change in visual function.
Unilateral or bilateral treatment (Recommendation 5)
There is no evidence from the pivotal clinical trials that unilateral or bilateral treatment influences the commencement or retreatment of patients (RESTORE, RIDE, RISE, Protocol I and T, DA VINCI and VISTA and VIVID studies). Similarly, there is no evidence that a better or worse seeing eye is treated differently. A compromise in treatment regime may have to be made for those receiving bilateral treatment due to logistical reasons.
Ocular co-morbidities (Recommendation 6)
Should a patient have an ocular co-morbidity, physicians need to consider the potential to restrict the effect of any treatment or cause more profound loss. An example may be co-existing foveal-involving atrophy due to age-related macular degeneration. Key studies excluded patients with significant co-morbidities from being enrolled into clinical trials.8,9,14,19 VA measurements therefore may not reflect change in DMO due to co-morbidities.
Systemic factors that influence the management of diabetic macular oedema with anti-vascular endothelial growth factor therapy
Blood glucose control (Recommendation 7)
The evidence that management with anti-VEGF therapy for DMO should differ based upon their glycated haemoglobin (HbA1c) is weak. An example of this is a post hoc analysis of the RIDE and RISE trials, in which the treatment benefit of ranibizumab for patients with DMO seemed independent of baseline HbA1c.20 A retrospective consecutive case series of 124 patients with DMO treated with anti-VEGF, however, did find that glucose regulation can impact on the response to anti-VEGF therapy.21 Patients with improved glucose control during the study had a significantly lower retinal thickness than patients who had a stable or worsening HbA1c (mean final CST of 324.3 versus 390.0 μm, respectively; p=0.042). Many studies have excluded patients with a very high HbA1c, including the RESTORE,9 RISE/RIDE,14 DRCR.net Protocols I8 and T10, the DA VINCI7 and VISTA and VIVID studies15 (see Table 4) and so the outcome in this group is not as well known.
Advising and reinforcing the importance of good glucose control at the point of contact during DMO management is good practice for the holistic care of patients with diabetes.21–23 Intensive glycaemic control decreases the rate of development and progression of diabetic retinopathy (DR) in type 1 and 2 diabetic mellitus patients. In the primary prevention cohort of the Diabetes Control and Complications Trial (DCCT), there was a 76% reduction in the adjusted mean risk for retinopathy development for patients who received intensive therapy. In the secondary intervention cohort, the progression of retinopathy was reduced by 54%, and the development of proliferative or severe non-proliferative retinopathy was reduced by 47%.24 The National institute for Health and Excellence (NICE) has suggested a target HbA1c for patients with diabetes of 48 mmol/mol (6.5%).25,26 It is also good practice to ensure appropriate arrangements are in place, with either a diabetologist or primary care physician for general diabetic care.
Blood pressure control (Recommendation 8)
There is little evidence that patients should be treated differently based upon their starting BP. The only potential exception is in those with very high BP who were excluded from the major clinical trials and therefore it is not known if their outcome differs (see Table 4).7–10,14 Likewise, once treatment has been commenced there is no evidence, based upon these studies, that retreatment should differ depending upon BP.
However, theoretically, higher BP will be associated with an increase in retinal capillary hydrostatic pressure due to the dysfunction of vascular autoregulation in the retina of patients with DR. According to Starling’s rules this will augment macular oedema, and indeed there is clinical and experimental evidence that high BP is an independent direct and shortterm driver of DMO.27–29
In addition, there is solid evidence to support the benefit of tightly controlled blood pressure (BP) on overall retinopathy status. The UKPDS investigated the role of tight BP control on various end points, including retinopathy and visual loss. In the group with tight BP control, a 35% reduction in the progression of retinopathy was observed compared with the group assigned to less tight control. At 9-year follow-up, the group with tight BP control had a 47% reduction in risk of loss of three or more lines of vision (using ETDRS chart).29 Gallego et al. identified that an increase of 10 mm/Hg in systolic BP was associated with a 3–20% increased risk of retinopathy and an increase of 10 mm/Hg in diastolic BP increased risk by 2–30%.30 As such, and based on clinical observations and the theoretical issues raised above, it is good practice to ensure BP is being addressed by the appropriate care physician and to recommend BP control in line with recommendation from organisations such as NICE and Diabetes UK (BP target: 130/80 mmHg if retinopathy, renal or cerebrovascular damage).25
Serum lipid control (Recommendation 9)
Based upon the pivotal anti-VEGF DMO treatment studies, there is no evidence that serum lipid status should alter the way in which anti-VEGF therapy is given. Neither inclusion criteria for the commencement of therapy or retreatment strategies were guided by serum lipid levels.
Although some study findings suggest that the management of serum lipids may reduce retinopathy progression and the need for treatment,31,32 other clinical data have disputed the association between lipids and the severity of DR or DMO. In an ETDRS report, high total cholesterol and low-density lipoprotein (LDL) levels were associated with retinal hard exudates.32 The Multi-Ethnic Study of Atherosclerosis and the Australian Diabetes, Obesity, and Lifestyle Study observed no association between serum lipids and DR.33,34 In another study, the level of total cholesterol, triglyceride, high-density lipoprotein (HDL), LDL and very low density lipoprotein (VLDL) was not significantly associated with the severity of DR or existence of macular oedema.35 Furthermore Chew et al. observed that DMO was not significantly associated with high triglyceride levels, total cholesterol or LDL levels when lipid profile was stratified.36
Evidence from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) and The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye studies have shown that fenofibrate may influence DR. In the FIELD study, fenofibrate (200 mg/day) reduced the need for laser therapy and prevented disease progression in patients with pre-existing DR.37,38 A systematic review and meta-analysis on dyslipidemia and DMO was inconclusive in its findings, indicating that further investigation into the relationship between lipid levels and DMO is needed.39
Even if controversy exists about the role of serum lipids for DMO specifically, it is important to provide advice on its control for holistic patient benefit. The current NICE guidelines for lipid modification have recommended that a full lipid profile assessment for people with diabetes should include measurement of total cholesterol, HDL cholesterol, non-HDL cholesterol and triglyceride concentrations.40 Diabetes UK recommends total cholesterol level <4.0 mmol/l, LDL levels <2.0 mmol/l, HDL levels ≥1.0 mmol/l in men and ≥1.2 mmol/l in women and triglyceride levels ≤1.7 mmol/l.41
Co-existing thiazolidinedione medication
(Recommendation 10)
Peripheral oedema and fluid retention associated with thiazolidinediones (TZDs) may increase the risk of DMO,42 however, the exact mechanism underlying this risk remains unclear but may be related to an independent or secondary effect from related systemic fluid retention, possibly by causing increased retinal capillary hydrostatic pressure and oedema formation by Starling’s rules. It is advisable to suspend TZD administration when macula oedema develops, since this action is likely to improve the condition. This should be done as a recommendation to the prescribing physician so alternative medication or strategies can be sought. It remains an option to observe whether DMO persists following suspension of a TZD and whether anti-VEGF therapy need be commenced.
Body mass index (Recommendation 11)
Some studies have demonstrated a relationship between obesity or higher body mass index (BMI) and an increased risk of DR.43–48 Kaštelan et al. suggested that BMI, in conjunction with poor glycaemic control, hypertension and dyslipidaemia, was associated with DR progression in patients with type 2 diabetes.43 Dirani et al. suggested that patients with diabetes with higher BMI and larger neck circumference were more likely to have DR and more severe stages of DR.45 NICE and the UK National Health Service (NHS) Choices guidelines advise that BMI should be maintained within the range 18.5 to 24.9 and that BMI values above 25 increase the risk of type 2 diabetes.49,40 There is, however, no evidence available suggesting that, in the context of anti-VEGF therapy, decreasing BMI improves response of DMO, or if it has an influence of how treatment should be administered.
Pregnancy or potential pregnancy (Recommendation 12)
Current evidence on the use of anti-VEGF therapy in pregnancy cases is very clear. Given the potential for teratogenicity, pregnancy is a contraindication for anti-VEGF therapy. There have been case reports, however, of unaffected babies born to mothers undergoing anti-VEGF therapy.51–54 Clear advice needs to be given on pregnancy prevention during anti-VEGF therapy or for 3 months thereafter. In addition, consideration needs to be given as to the appropriateness of within clinic pregnancy testing.
Stroke and myocardial infarction (Recommendation 13)
There continues to be discussion in the literature about the potential signal of increased risk of cerebrovascular or cardiovascular events with the use of anti-VEGF therapy. Studies do not enrol patients if these events have occurred recently (<3 months). The risk of recurrence of stroke and myocardial infarction when they have recently occurred (<3 months) in the context of anti-VEGF therapy has not been specifically studied and so careful discussion with the patient about the potential risk and benefits of such treatment is required in this circumstance.7,9,10,14,55
Psychosocial factors that influence the management of diabetic macular oedema with anti-vascular endothelial growth factor therapy There is little good quality evidence that psychosocial factors influence the use of anti-VEGF therapy in the management of DMO; therefore, the statements in this section are based on opinion.
Quality of life and patient-reported symptoms
(Recommendation 14)
QoL utilities and patient-reported symptoms were not used as selection or retreatment criteria in the RESTORE,9 RISE/RIDE,14 DRCR.net Protocols I8 and T10 or VISTA and VIVID studies.15
Age (Recommendation 15)
There is no high-level evidence supporting any association between the influence of adult age on the response to anti-VEGF therapy or alteration in how treatment should be administered. It is noteworthy that the age limitation in the key studies was ≥18 years, without an upper limit (see Table 4).9,10,14,19,55
Profession (Recommendation 16)
There are data demonstrating that a patient’s working profession is restricted and their employment is made more expensive due to visual impairment by DR.2,56–58 There are, however, no data to suggest that a patients’ profession has any influence on DMO diagnosis or management.
Compliance (Recommendation 17)
Anti-VEGF therapy in non-compliance patients was not studied in any of the work identified by the literature search. Furthermore, trial participants are not usually included in key clinical trials if the recruiting physicians feel that they will not be compliant with study visits.
People with both vision-threatening DR and psychosocial problems may have significantly reduced levels of functioning compared with psychologically healthy counterparts.59 This may have a negative impact on compliance, diabetes control and subsequent outcome. Pivotal studies have not specifically studied this group.
Conclusions
The evidence for the major drivers influencing the management of DMO with anti-VEGF therapy are undoubtedly ocular specific: VA and OCT findings. The panel discussed how patients may decide not to commence therapy though, even in the presence of reduced acuity and a thickened OCT macular scan. This is not an uncommon scenario particularly if a patient was asymptomatic or does not consider the symptoms to be sufficiently troublesome. The evidence for not commencing therapy, or indeed deferring therapy with or without macular laser for a significant period of time (74 weeks or more), demonstrates that VA outcomes are inferior compared with those commencing therapy early.60 Although there is little direct evidence that control of systemic factors such as HBA1c, BP and dyslipidemia influence how therapy should be given, there are signals that in the short- and long-term (HBA1c and dyslipidemia) controlling these factors can help with the control of DMO and DR. It is important, not only from this point of view, but also for the holistic care of patients to recommend optimal systemic risk factor control. This should be achieved through communication with the physician in charge of the blood glucose management whether they are a primary care physician or diabetologist.
Psychosocial factors are important in influencing how patients are managed. Ultimately it is symptoms and QoL that physicians are trying to improve and indeed often are guided by these to influence commencement of treatment. As symptoms and QoL utilities can be either imprecise or relatively crude measures, they are not used as primary treatment endpoints in clinical trials and so no evidence exists to support their use as specific treatment endpoints. Visual function QoL has been shown to improve in key studies though, although EuroQol five dimensions questionnaire (EQ-5D) did not improve in the RESTORE study. The means by which psychosocial factors influence how patients are managed is so far poorly studied.
There are a number of potential factors that influence the way in which the DMO patient is managed, but many lack a firm evidence base. This lack of evidence raises the risk that treatment decisions may be partly based on assumptions. Better and more extensive evidence to support DMO treatment remains an unmet need. Physicians treating DMO are therefore pointed towards a list of key ocular-specific, systemic and psychosocial considerations that are vital in deciding when to commence or retreat a patient with DMO (see Table 2) and these may help improve outcomes.







