The eyelids may harbor malignancies that can pose serious risks to the eyes. Some carcinomas can be life threatening or associated with distant metastasis. More commonly, failure to diagnose and treat in a timely manner can lead to local tumor growth, with possible direct extension to the adjacent ocular surface, orbit, or skull base. Late treatment may require extensive resection and reconstruction. Early detection of eyelid cancer is key. Distinguishing benign from malignant periocular lesions can be challenging. A patient’s past medical history, as well as an assessment for characteristic signs, helps lead to a suspicion for malignancy, a decision for biopsy, and a definitive diagnosis. In this review, the authors describe common clinical signs of eyelid malignancies and detail features of some of the more common carcinomas found in the periocular region. We also describe treatments, including novel targeted therapies, for some aggressive periocular cancers.
Patient history
The timeline of symptoms may aid in distinguishing a new, rapidly growing lesion from a longstanding lesion with recent transformation. Other symptoms, such as bleeding, crusting, non-healing, or recurrence, should prompt further work up. Risk factors for cutaneous eyelid malignancies include advanced age, significant sun exposure with sunburn or prior skin cancer, prior radiation therapy (RT), or immunosuppression. A personal history of prior skin cancer and/or a family history of skin cancer, particularly melanoma, may be important.
Characteristics of eyelid malignancy
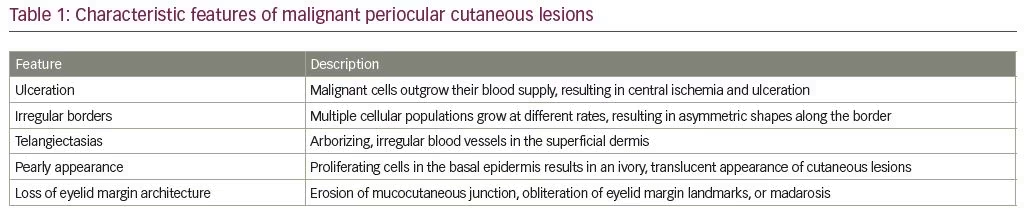
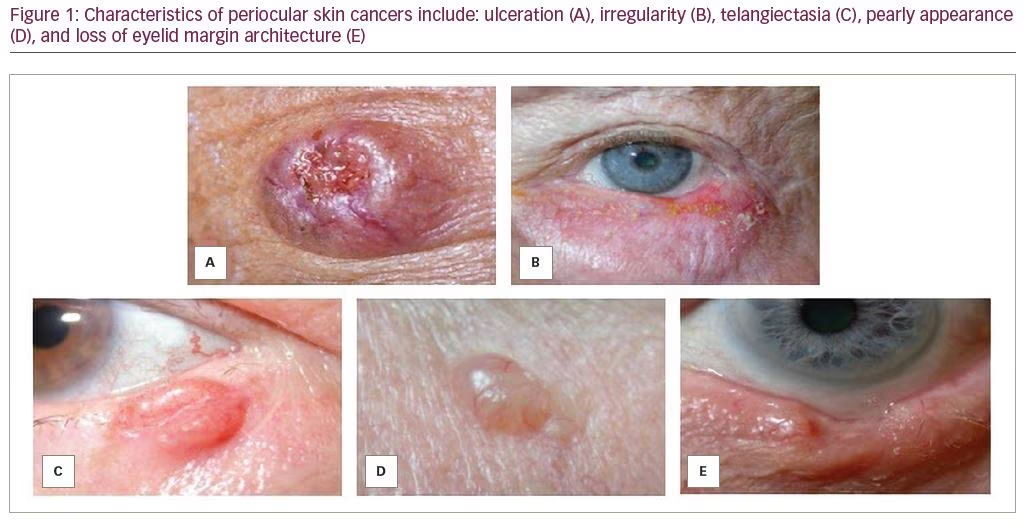
Ulceration, irregular borders, telangiectasia, pearly borders, and loss of eyelid margin architecture (Table 1) are key features suggestive of eyelid malignancy. In particular, these findings are associated with periocular non-melanoma skin cancers, namely basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).1 Asymmetry, border, color, diameter, and evolution (ABCDE), are the classic features to ascertain whether pigmented skin lesions are a concern for melanoma.2
Ulceration
Ulceration develops as malignant cells grow haphazardly, and are thought to outgrow their own blood supply (Figure 1A). With nodular BCC, tumors are perfused peripherally; however, central ischemia and ulceration develop. In SCC, ulceration may be seen at the periphery of the lesion. In melanoma, ulceration suggests a more aggressive subtype or advanced disease. Ulceration—particularly that which is chronic and recurrent—should prompt biopsy, as this feature is rarely seen in benign lesions. One exception is molluscum contagiosum, a benign viral lesion that may present with chronic ulceration at the site of central umbilication.
Irregularity
Irregularity in the contour or borders of an eyelid lesion occurs due to the presence of multiple cellular populations growing at different rates and should raise suspicion for malignancy (Figure 1B). Malignant tumors often have scalloped margins with asymmetric shapes, whereas benign lesions, such as syringomas, epidermal cysts, and intradermal nevi, typically have smooth, discrete borders with a symmetric shape.
Telangiectasia
Telangiectasias are dilated, irregular, small blood vessels of the superficial dermis (Figure 1C). They are present in sun-exposed areas of the skin in older, fair-skinned individuals and are associated with numerous cutaneous pathologies, including rosacea and scleroderma. Dermatoscopic studies have shown that the pattern of arborizing telangiectasias is consistent with nodular BCC on the face compared to the spoke-wheel, fine telangiectasias associated with superficial BCC.3 While isolated telangiectasias are commonly seen on older, sun-damaged skin, telangiectasias overlying raised, scaly, or irregular skin lesions should raise suspicion for an underlying skin cancer.
Pearly appearance
A whitish and shiny appearance of a lesion may be suspicious. In particular, nodular BCC presents with a scalloped pearly border and translucent appearance secondary to proliferating cells in the basal epidermis (Figure 1D). The differential diagnosis of pearly appearance may also include benign lesions, such as amelanotic nevi, papilloma, or molluscum contagiosum, among other less common entities.
Loss of eyelid margin architecture
Erosion of the mucocutaneous junction, a focal depression or notch, or other obliteration of eyelid margin landmarks, such as the meibomian gland orifices or lash line, are suspicious (Figure 1E). Madarosis, or loss of the eyelashes, is common with malignancy due to destruction of hair follicles, particularly with lesions that disrupt the normal eyelid margin architecture. As the tumor outgrows its blood supply, necrosis may ensue with cycles of granulation and healing. Eyelid margin destruction is particularly characteristic of morpheaform BCC or SCC and should encourage prompt biopsy.
Basal cell carcinoma
BCC is the most common cutaneous malignancy, comprising 90% of eyelid malignancies and approximately 20% of all eyelid tumors in general.4 Risk factors include ultraviolet (UV) exposure, specifically intermittent intense UVB, and fair skin. The association with sunburns suggests intensity of light rather than prolonged sun exposure and predisposes individuals to BCC. Nodular BCC may lead to what has been described as a “rodent ulcer,” due to a destructive, “eaten” appearance, which comprises half of all BCCs. The classic appearance, especially early on, is a pink, pearly papule with a smooth translucent surface and telangiectasias. Additional features include margins that are difficult to discern, an indurated texture, and central ulceration. Common high-risk features include large lesion size and proximity to the medial canthus.5,6 Pigmented BCCs are a brown or black-blue hue and are distinguished from melanoma on histopathology. Morpheaform BCC is more invasive with a worse prognosis.6 These lesions may mimic a scar and have less discrete borders and skip lesions.
If clinical features are suspicious for malignancy, a biopsy should be performed. Incisional biopsy is useful for large lesions that will require subsequent wide local resection with or without Mohs micrographic surgery (MMS) followed by reconstructive surgery. Otherwise, smaller lesions may be amenable to excisional biopsy with generous margins, permanent sections, and concurrent reconstruction to avoid multiple surgical sessions.
BCC most commonly involves the lower eyelid due to greatest direct sun exposure, followed by the medial canthus, upper eyelid, and lateral canthus.7 If the lesion involves the medial canthus, magnetic resonance imaging (MRI) of the orbits should be considered to evaluate for orbital extension.8
Squamous cell carcinoma
SCC is less common than BCC, comprising 5–10% of eyelid malignancies with a BCC to SCC ratio of 4:1.6,9 SCC occurs in sun-damaged skin and may arise de novo or from actinic keratosis. The latter results from atypical keratinocyte proliferation and is classified as a pre-malignant lesion; thus, it should be monitored closely for characteristics suggestive of malignant transformation. Keratoacanthoma, also a precursor to SCC, is characterized by rapid onset (2–3 weeks) of a crater-like lesion with central ulceration and raised erythematous borders.10,11 Seborrheic keratosis is a “greasy,” “stuck-on”
lesion on the differential for SCC, but is benign. These lesions are typically seen in elderly patients and can be removed if they are causing irritation.
SCC may appear clinically as scaly skin, cutaneous horns, and rough patches.12,13 Additionally, ulceration, telangiectasias, and erythema may be present along the margins and should prompt biopsy.6 On histopathology, classic findings include hyperkeratosis and keratin deposits. SCC is typically more aggressive than BCC and, when in advanced stages, may have perineural invasion, which is challenging to completely resect.14,15
Sebaceous cell carcinoma
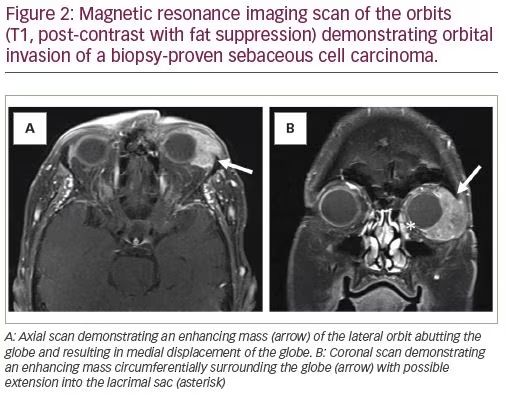
Sebaceous cell carcinomas (SBCC) are rare, comprising <1–5% of eyelid cancers.16 These are aggressive tumors that arise within sebaceous glands of the eyelids and are potentially lethal. They have no characteristic appearance, but may present as chronic blepharoconjunctivitis or a recurrent chalazion.17 Therefore, any chalazion recurring in the same location should be investigated. A full-thickness biopsy is usually required to make the diagnosis. The sample is usually sent as a fresh specimen; permanent fixation with formalin may wash the lipoid component and tarnish the diagnostic yield. SBCC can have skip lesions; hence, a wide resection should be performed. Some experts recommend conjunctival map biopsies to assess for skip lesions, particularly in cases with multifocal masses on gross examination.18 Sentinel lymph node biopsy (SLNB) can be considered, although the effect on survival outcomes is unclear.19 Orbital exenteration is necessary in some aggressive cases with orbital extension to prevent the risk of intracranial extension and metastasis (Figure 2).
Melanoma
Eyelid melanoma is rare, making up 1% of all malignant neoplasms of periocular skin.20 It occurs in one of three forms: superficial spreading malignant melanoma, lentigo maligna melanoma, and nodular melanoma. Lentigo maligna melanoma is typically non-palpable, with tan to brown pigmentation and irregular margins; however, darker and elevated lesions occur with dermal invasion. Nodular melanoma is a slightly raised blue-black lesion but can also be amelanotic. Malignant melanoma has potential for distant metastasis to areas including the central nervous system, bone, and gastrointestinal tract, and is often lethal.21,22 The risk and prognosis correlate directly with Breslow depth, which can be obtained through early incisional biopsy of pigmented lesions demonstrating changes in size, color, or shape. Metastasis occurs through lymphatic spread; therefore, regional nodal status is the most important prognostic factor.23,24 Consideration should be given to performing SLNB concurrently with surgical excision, particularly in lesions with Breslow thickness greater than 0.8 mm or with ulceration.25,26 For intermediate thickness melanoma, SLNB has been associated with improved survival in controlled studies. The differential diagnosis for pigmented lesions includes benign lesions, such as nevi, verruca, papillomas, and solar lentigos. These lesions can be irritating or pruritic in contrast to the indolent presentation of melanoma.
Merkel cell carcinoma
Merkel cell carcinoma (MCC) is rare, but increasing in frequency due to improved diagnosis and detection. MCC is a highly malignant neuroendocrine tumor with worse prognosis in the head and neck compared with other sites.27 MCC is associated with age greater than 50 years and immunosuppression, including human immunodeficiency virus and organ transplantation. The tumor presents as a painless, rapidly growing, non-tender, red or violaceous nodule, with difficult to discern borders. It is aggressive, with high incidence of regional metastasis. Therefore, wide tumor resection with 1–2 cm margins is recommended, and SLNB may be considered to detect regional metastasis.27
Biopsy techniques
Once a lesion is selected for biopsy, the sample can be obtained using incisional or excisional biopsy techniques. During collection, care should be taken to avoid excessive traumatic injury to the sample (e.g. crushing or cauterizing).
Incisional biopsy
An incisional biopsy involves removal of part of the lesion through a shave or punch biopsy technique. If the lesion is confirmed to be benign, an incisional biopsy is advantageous as it produces a good esthetic result with minimal surgical intervention and minimal distortion of the adjacent tissue. Further, it is advantageous for larger, high-risk lesions, as it allows quick and convenient tissue sampling to aid further surgical planning and coordination among specialists (e.g. a Mohs surgeon or surgical oncologist). Conversely, the disadvantage of incisional biopsy is that it requires an additional surgical procedure if the final histopathology reveals malignancy.28 Nevertheless, incisional biopsy allows sampling of the lesion and can usually be performed in a timely manner as an in-office procedure under local anesthesia.
Excisional biopsy
Excisional biopsy involves the entire removal of all grossly visible tumor.29 For eyelid lesions, full thickness excisional biopsies may completely treat lesions close to the eyelid margin. Medial eyelid tumors should be resected with caution due to their proximity to the canaliculi. Concurrent nasolacrimal stenting and repair of the canaliculi may be warranted. For excisional biopsy of a non-superficial eyelid margin lesion, a full thickness pentagonal wedge excision with appropriate margins may achieve excellent esthetic and functional results. The apex should extend through the entirety of the tarsus to avoid eyelid contour abnormalities including notching and kinking.30 Despite careful approximation of the eyelid edges, the vertical wound results in a conspicuous scar and the triangular apex can have skin redundancy or form a “dog-ear.” A curvilinear, or “lazy” pentagonal wedge excision design may achieve tarsal alignment while reducing cutaneous redundancy and keep the incision aligned with the relaxed facial skin tension lines.31
Diagnostic studies
Adjunctive diagnostic studies may aid in diagnosis and staging of advanced cutaneous malignancies and should be obtained in specific circumstances. Pre-operative neuroimaging of the orbits with MRI or computed tomography (CT) should be obtained if the affected eye has abnormal conjunctival fornix architecture or if orbital signs are present, including vision changes, restriction of extraocular movements, proptosis, or pupil abnormality.
MRI is more sensitive for evaluating the orbital soft tissues—the primary pattern of invasion for most eyelid malignancies. However, CT is useful if bony erosion is suspected. CT is also indicated if there is a contraindication to MRI, such as a metallic implant, or if MRI is not readily available. For highly aggressive tumors with potential for regional metastasis, such as SBCC or MCC, positron emission tomography may be indicated to highlight areas throughout the body of high metabolic activity which are suggestive of metastases.
Treatment options
Treatment options for biopsy-proven eyelid and periocular malignancies vary based upon the malignancy and depth of invasion. As mentioned earlier, excisional biopsy itself may be curative. For biopsies with positive tumor margins, wide local excision with confirmation of negative surgical resection margins is the gold standard. Cryo-, thermo-, or local chemotherapy are options, especially if a patient cannot tolerate surgery. However, these options are less desirable owing to proximity to the eye. Importantly, tumor eradication is not histologically validated with these tissue destruction options. Hence, surgical resection with intraoperative frozen sections or MMS to evaluate margins prior to reconstruction in the same or separate surgical setting is preferable. Incomplete tumor excision remains a small risk, even with MMS or other frozen section assessment.32
For aggressive tumors, especially if the margins remain positive, globe-sparing medical oncology treatment options should be considered when appropriate. Neoadjuvant, adjuvant, and radiation therapies may not eradicate disease, but may reduce tumor size burden to improve the opportunity for surgical resection.
When globe-sparing options are futile, or if the globe and vision are already compromised, locally advanced tumors confined to the orbit can be treated with orbital exenteration. In these procedures, periocular and orbital contents, including the eyeball, orbital soft tissues, lacrimal gland, lacrimal drainage apparatus, periosteum, and eyelids are removed en bloc. This procedure is potentially lifesaving, despite the disfiguring side effects.
Surgical oncology specialty involvement may be useful for local tumor resection or concomitant SLNB or both, particularly in cases of SBCC, melanoma, Merkel cell carcinoma, or invasive SCC with high-risk features. However, the role of SNLB in malignancies, besides intermediate-thickness melanoma, is yet to be defined.6,19
Targeting cell signaling pathways involved in eyelid tumor pathogenesis is a new paradigm for certain invasive cancers, particularly those that are not amenable to surgical resection or when orbital exenteration can be avoided.33
Targeting the aberrantly activated sonic hedgehog signaling pathway has been associated with improved clinical response in patients with advanced BCC. Vismodegib deactivates the Sonic hedgehog signaling pathway through inhibition of the Smoothened receptor.33 It is currently indicated for BCC that is locally advanced, metastatic, recurrent, or not amenable to surgery or RT.34 Moreover, when used in combination with mammalian target of rapamycin inhibitors and RT, surgical resection has been found to be less extensive.35 Its use as neoadjuvant therapy with the goal of globe-sparing surgery is under investigation.
Overexpression of epidermal growth factor receptor (EGFR) has been shown in SCC pathogenesis. Thus, targeted therapies for non-resectable, metastatic, or recurrent SCC of the head and neck include EGFR inhibitors and immune checkpoint inhibitors. Erlotinib, which has been approved for use by the US Food and Drug Administration in advanced non-small cell lung and pancreatic cancers, is a tyrosine kinase EGFR antagonist used off-label for advanced periorbital SCC.33 Cetuximab is a monoclonal antibody EGFR antagonist used in combination with RT for locoregionally advanced tumors or with platinum-based chemotherapy for recurrent/metastatic cutaneous SCC. It has also been used as neoadjuvant therapy to chemo-reduce tumor burden and improve surgical outcomes.36 Immune checkpoint inhibitors include pembrolizumab and cemiplimab. Pembrolizumab, a programmed cell death protein 1 (PD-1) receptor inhibitor, has been associated with regression of a massive, non-resectable SCC.37 Migden and colleagues report that in patients with advanced cutaneous SCC, PD-1 blockade with cemiplimab showed a response rate of 47% in patients with metastatic disease.38 The role of these treatments may be incompletely understood currently, but these therapies demonstrate a potential to augment or possibly replace aggressive surgical resection.
Cutaneous melanomas have demonstrated clinical response with targeted molecular therapy. Fifty percent of melanomas have BRAF gene mutations with 90% of these cases being the V600E gene mutation, which leads to persistent activation of the mitogen-activated protein kinase (MAPK) signaling pathway. Vemurafenib is a BRAF inhibitor used for metastatic or non-resectable melanoma with the V600E mutation. Dabrafenib, a BRAF inhibitor, is used with trametinib, a MEK inhibitor, for V600E and V600K mutations, as resistance develops through activation of the MEK signaling pathway.39 Currently, there are no reports of BRAF inhibitors for the treatment of eyelid melanomas. However, conjunctival melanoma has demonstrated improved clinical outcomes with promising long-term results.40 Immune checkpoint inhibitors for cutaneous melanoma include nivolumab (PD-1 inhibitor) for metastatic or non-resectable melanomas, pembrolizumab for metastatic melanoma, and ipilimumab (CTLA-4 inhibitor) for metastatic and non-resectable melanoma.
The primary treatment for SBCC is wide surgical excision, although pembrolizumab has been used off-label for advanced stage or recurrent sebaceous carcinoma.41 For advanced and metastatic MCC, avelumab—an anti-PD ligand-1—has demonstrated clinical response.42 Pembrolizumab and nivolumab also have been shown to improve survival in advanced and metastatic MCC.42 However, no specific studies have investigated their efficacy in eyelid or periocular MCC.
Conclusion
Eyelid cancer is common, with BCC being the most prevalent.4,6 Ulceration, irregularity, telangiectasia, pearly appearance, and loss of eyelid margin architecture are key features suspicious for malignancy. The final diagnosis rests with tissue biopsy. Surgical excision with negative margins remains the gold-standard therapy, although newer therapies are available for advanced cases or when surgical resection is not possible. Targeted therapies are promising for aggressive or advanced periocular cancers.