Leading Multinational Ophthalmology Clinical Research in Europe

EVICR.net is a network of European Ophthalmological Clinical Research Centres, dedicated to performing multinational clinical research in ophthalmology with the highest standards of quality, following European regulations for clinical research and the International Conference on
Harmonisation Good Clinical Practice (ICH-GCP) guidelines. At present, EVICR.net has 100 Clinical Ophthalmological Centre members from 15 European countries. The network has already coordinated 20 multinational clinical studies and has at present eight clinical studies ongoing, of which three are funded by the European Commission.
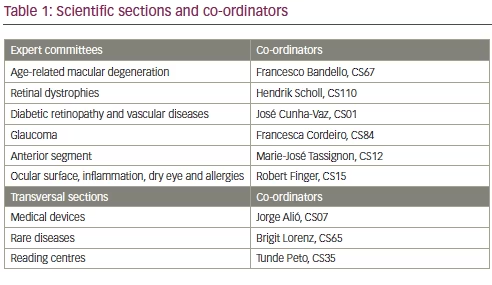
The present EVICR.net chairman is Hendrik Scholl, Professor and Chairman of the Department of Ophthalmology of the University of Basel, Switzerland, and the director of the Institute of Molecular and Clinical Ophthalmology, Basel, Switzerland. Scientifically, EVICR.net is organised by ophthalmology subspecialty expert committees and it also has transversal sections. Table 1 shows details the different scientific sections and the corresponding co-ordinators.
In Europe, multinational investigator-initiated research (IIR) is still a challenge, as one must overcome country borders, different national requirements and different languages. These studies require overall co-ordination that cannot feasibly be performed by investigators alone. EVICR.net is the perfect partner for the co-ordination of multinational IIR, particularly when applying for European Commission funding or IIR grants from industry. EVICR.net members have the opportunity to participate in IIR within the network, as well as to submit abstracts for IIR that are evaluated by a specific expert committee. When approved, members will have access to support for the overall co-ordination and implementation of the study.
EVICR.net IIR has been expanding in recent years, providing the opportunity for investigators to perform multinational clinical research of high quality, in compliance with ICH-GCP guidelines. EVICR.net’s relevant role in the co-ordination of clinical studies within European Union-funded
projects has been highlighted as an instrumental asset for the implementation of multinational clinical studies, such as MACUSTAR and RECOGNISED, with a track record of experience in successfully conducting multicentre clinical research studies.

MACUSTAR – Intermediate AMD: Development of novel clinical endpoints for clinical trials in patients with a regulatory and patient access intention (IMI2-116076)
The objectives of MACUSTAR are to characterise the functional deficit in intermediate age-related macular degeneration (iAMD), and to develop and validate functional, structural and patient-reported outcome measures for iAMD. In addition, risk factors for progression from iAMD to late AMD will be investigated. The Coordinating Investigator is Prof. Frank Holz and the sponsor is the University Hospital Bonn in Germany. MACUSTAR’s core is an observational multinational clinical study with 20 EVICR.net clinical sites from seven European countries.
This project has received funding from the Innovative Medicines Initiative 2 joint undertaking under grant agreement No. 116076. This joint undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations.
![]()
RECOGNISED – Retinal and cognitive dysfunction in type 2 diabetes unravelling the common pathways and identification of patients at risk of dementia (H2020-SC1-BHC-01-2019-847749)
The aims of RECOGNISED are to investigate the common mechanisms involved in the pathogenesis of diabetic retinopathy and cognitive impairment in type 2 diabetes. The co-ordinating investigator is Prof. Rafael Simó and the sponsor is Vall d’Hebron Institut de Recerca in Barcelona, Spain. This project involves 11 RVICR.net clinical sites from seven European countries.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 847749.
EVICR.net also has an educational programme with the aim of creating educational webinars on ophthalmology clinical research, developed by well-known experts in their scientific areas sharing their experience and expertise with the clinical research community. EVICR.net participates in the European Reference Network on Rare Eye Diseases (ERN-EYE) as a member of the Scientific, Medical and Ethical Advisory Board, and by providing overall management and logistical support needed in multinational IIR in rare eye diseases. Many ERN-EYE members are also EVICR.net members.
EVICR.net is an Affiliate Partner of European Clinical Research Infrastructure Network (ECRIN-ERIC). This means that in the area of vision and ophthalmology multinational clinical research, ECRIN-ERIC will partner with EVICR.net, particularly in providing scientific and medical expertise, access to patients and clinical research capacity through its members. Thus, EVICR.net strengthens the capacity of the European Union to perform multinational clinical research studies to develop and
optimise the use of diagnostic, prevention and treatment strategies in ophthalmology, bringing better patient care to daily clinical practice.
EVICR.net offers a unique platform for ophthalmology multinational clinical research in Europe and is a useful industry resource in order to contribute to the development of new drugs, gene and cell therapy products, medical devices and biomarkers.
Contacts
Cecília Martinho https://www.linkedin.com/in/cecilia-martinho-9909012/
Daniel Fernandes https://www.linkedin.com/in/danielsanchesfernandes
EVICR.net Coordinating Centre
AIBILI, Azinhaga de Santa Comba, Celas, 3000-548 Coimbra, Portugal
Tel.: +351 239 480 101/15, E: evicrnet@aibili.pt, W: www.evicr.net




