Retinal imaging serves as a critical adjunct to the diagnosis, monitoring, and treatment of numerous ocular diseases. Since the invention of the direct ophthalmoscope by Hermann von Helmholtz in 1851, techniques to visualize the fundus have grown exponentially both in number and sophistication. Notably, wide-field retinal imaging has taken center stage over the past 2 decades. Given its increasing popularity in research and clinical use, it is important to be aware of the different types of wide-field imaging currently available, as well as the clinical applications for, advantages, and limitations of widefield retinal imaging. This review article aims to highlight our current understanding of wide-field retinal imaging and provide an outlook for its future implications.
Historical Perspective
The first fundus camera was developed by Frederick Dimmer at the turn of the 20th century. Dimmer was the first to incorporate fundus photography into an ophthalmic textbook, and he published the first photographic atlas in black-and-white.1 According to Dimmer’s original description, his camera occupied an entire tabletop and only one such camera was ever produced. The first modern fundus camera was created by JW Nordenson in 1925 and became commercially available through the Carl Zeiss Company in 1926. Based on Gullstrand’s principles, the Nordenson-Zeiss camera created a 10° field of view, color fundoscopic image. It was widely used and remained the predominant system until 1953, when the electronic flash was adapted to the fundus camera.
Using the Zeiss-Nordenson camera, the first photographic technique of fluorescein retinal angiography was reported in 1958 when P Chao and M Flocks investigated a method for studying the retinal circulation time in cats.2,3 This laid the basis for the work of HR Novotny and DL Alvis, who in 1961 published their technique of fluorescein angiography (FA) in human eyes and subsequently have been considered the originators of this imaging modality. Donald Gass popularized its use in the 1960s, using FA to recognize previously unknown manifestations of existing diseases and to discover new clinical entities. FA has become a mainstay in the diagnosis, management, and treatment of a host of diseases, especially retinal vascular diseases and uveitis.
The Advent of Wide-field Imaging
Conventional retinal imaging currently utilizes a fundus camera that limits the field of view to approximately 30 to 50° of the retina in a single capture. While these imaging techniques allow for adequate visualization of the optic nerve and posterior pole, they are limited in providing views of the peripheral retina due to restraints imposed by the inherent spherical properties of the eye. Several diseases, such as Coats’ disease and sickle cell retinopathy, manifest principally in the peripheral retina, making imaging of these entities more difficult.
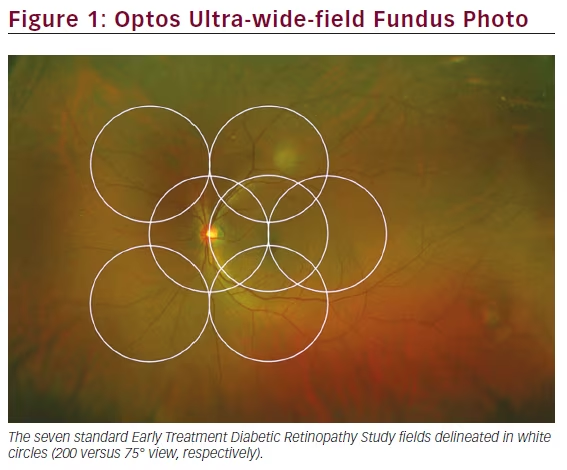
In 1975, Pomerantzeff developed the Equator-Plus camera, the first wideangle camera system, which used a contact lens and fiber optic scleral transillumination to obtain a 148° field of view.5 In the early 1990s, the Early Treatment Diabetic Retinopathy Study (ETDRS) detailed a systematic method of acquiring images of the retinal periphery in order to grade the presence and severity of diabetic retinopathy.6,7 In these trials, a series of seven individually acquired, stereoscopic, 30° retinal photographs were captured for each eye with traditional lenses according to a standardized protocol (see Figure 1). These photos were then viewed independently or manually fused into a montage to create an image that, in aggregate, covered approximately 75° field of view, or up to the midperiphery of the retina. This set of seven-field ETDRS photos became widely accepted as the gold standard for detecting and classifying diabetic retinopathy for the purposes of clinical trials.
Current Wide-field Imaging Systems
In addition to creating montages, there are two additional methods for obtaining peripheral views of the retina: 1) using a special lens in conjunction with a small-angle camera and 2) using a dedicated wideangle camera system. Both contact and noncontact accessory lenses have been used to increase the field of view of fundus cameras since the early 1980s.
First introduced in 1997, the Retcam (Clarity Medical Systems, Inc., Pleasanton, CA) uses a contact lens and fiberoptic cable connected to a computer monitor to image up to 130° field of view. Its main use is in the pediatric population to image diseases, such as retinopathy of prematurity (ROP) and Coats’ disease, as the system has a very low tolerance for any degree of media opacities. The Panoret-1000 (CMT Medical Technologies, Inc., Valley Stream, NY) was a portable, handheld fundus camera that utilized transscleral fiber optic illumination and was therefore less subject to media clarity. It was used in both the pediatric and adult population but involved a steep learning curve, was cumbersome to use, and is no longer commercially available.
In the early 1980s, confocal scanning laser ophthalmoscopy (cSLO) technology was developed based on the scanning laser microscope. This technology offers superior performance compared with conventional systems. cSLO uses monochromatic laser illumination combined with a confocal aperture to produce high-definition images. It effectively images patients with poor dilation, utilizes less bright light, thereby minimizing patient discomfort, and allows for 3D imaging and video capability.
The Optos camera (Optos PLC, Dunfermline, UK) became commercially available in 2000 and uses cSLO technology to image up to 200° of the retina horizontally (representing approximately 82.5 % of the total retinal surface) in a single capture without the need for adjunctive contact lenses or pupillary dilation (see Figure 1). To produce such “ultra” widefield images, the Optos platform harnesses the optical properties of an ellipsoid mirror and uses two wavelength lasers (red and green) to enhance visualization of retinal substructures, unlike conventional devices, which employ full-spectrum white light. The Optos device is also able to obtain red free, FA, indocyanine green angiography (ICG), and autofluorescence (AF) images.
In 2005, the Heidelberg-Staurenghi lens system combined the use of a cSLO-based imaging platform with a wide-field contact lens, the Ocular Staurenghi 230 SLO Retina Lens (Ocular Instruments, Inc., Bellevue, WA), to provide up to 150° field of view. In conjunction with the Heidelberg Spectralis imaging system (Heidelberg Engineering, Inc., Heidelberg, Germany), this unit can also obtain wide-field FA, ICG, and AF images. The disadvantage to this system is the contact lens requirement, which may be inconvenient to both the patient and photographer. While images captured with this system can be quite impressive, they require an experienced photographer and a cooperative patient in order to obtain them. A noncontact lens wide-field adaptation is also available from Heidelberg and provides a 55° field of view for AF and angiographic images.
Advantages and Disadvantages of Wide-field Imaging versus Traditional Fundus Cameras
Advantages
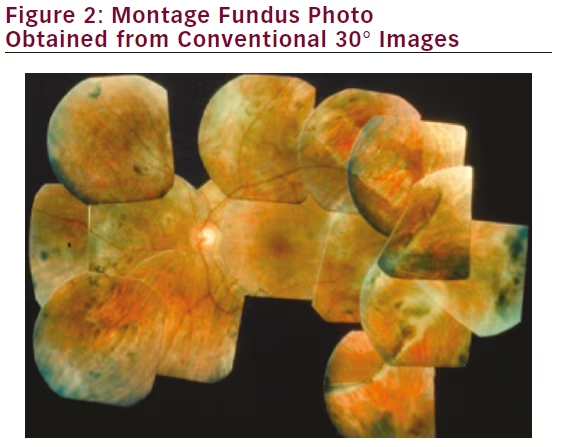
Attempts to image the retinal periphery with traditional fundus cameras require excellent pupillary dilation, relatively clear media, and good patient cooperation, as patients must move their eyes in specified directions in order to image the desired peripheral retinal areas. A skilled photographer is critical in order to obtain high-quality photographs, even for posterior pole images. In addition, the creation of montages is time-consuming, prone to errors, and heavily reliant on the imaging technician’s skills (see Figure 2). While digital imaging and computer software have made the acquisition of photos and montages easier, this technology is limited for dynamic imaging processes such as FA, as it is impossible to create a mosaic of images captured at exactly the same time point.
By contrast, wide-field imaging requires no pupillary dilation, is significantly more resilient to media opacities, and views extending from the posterior pole to the peripheral retina can be obtained in a single capture with the patient simply looking straight ahead. Many wide-field imaging systems offer FA, ICG, and AF in addition to color fundus photos, which allow the entire field of view to be visualized at determined time points.
Furthermore, while the standard ETDRS protocol comprises seven photos for each eye, a minimum of 32 photos are taken per patient in order to generate these final stereoscopic photos. With the capacity to image up to 200° of the retina in one photograph, wide-field imaging substantially reduces the time spent obtaining photographs and minimizes discomfort and flash exposure to the patient.
Disadvantages
Traditional fundus photography offers realistic, outstanding image quality, at times with higher resolution than current wide-field imaging systems. Optos imaging is subject to artifacts, including eyelashes and eyelids obscuring the superior and inferior portions of the fundus,8 and retinal colors are distorted due to only the red and green laser wavelengths used. Third, projecting a 3D globe as a 2D image inherently causes distortion in the apparent size of the images. While new software algorithms have been implemented to address the size-distortion issue on the Optos platform, this remains a persistent problem for other devices.
Several other possible disadvantages include the following: AF on widefield imaging may not be as robust as other platforms, the current software interface for wide-field imaging is still suboptimal, and cSLO ultra-widefield retinal imaging overall remains an expensive technology. Finally, current wide-field imaging does not capture from ora to ora, such that the anterior retina is visible only by binocular indirect ophthalmoscopy in most cases. In particular, the far superior and inferior peripheral retina are difficult to capture consistently with the Optos platform.
Clinical Applications of Wide-field Imaging
There are an increasing number of reports on the utility of wide-field imaging in a host of retinal diseases.
Diabetic Retinopathy
Diabetic retinopathy has long been recognized as a disease affecting the peripheral retina. Several studies have demonstrated that wide-field imaging can effectively illustrate the extent of diabetic retinopathy both via color fundus photos and FA both compared with traditional fundus photos obtained via the ETDRS protocol and clinical exams.9 One study showed that Optomap imaging enabled earlier diagnosis of higher grades of diabetic retinopathy compared with clinicians performing slit lamp biomicroscopy of the fundus.10 Similarly, wide-field imaging has been shown to capture more retinal nonperfusion, neovascularization, and panretinal photocoagulation than the seven-standard fields obtained by the ETDRS protocol (see Figure 3). Wessel et al. reviewed 236 Optos FAs from 118 patients with diabetes and found that ultra-wide-field FA revealed 3.9 times more nonperfusion, 1.9 times more neovascularization, and 3.8 times more panretinal photocoagulation than the seven standard fields.11 Ten percent of eyes had pathology beyond the simulated seven standard field boundaries, and in 17 % of eyes with neovascularization, the neovascularization was located outside the seven standard fields.
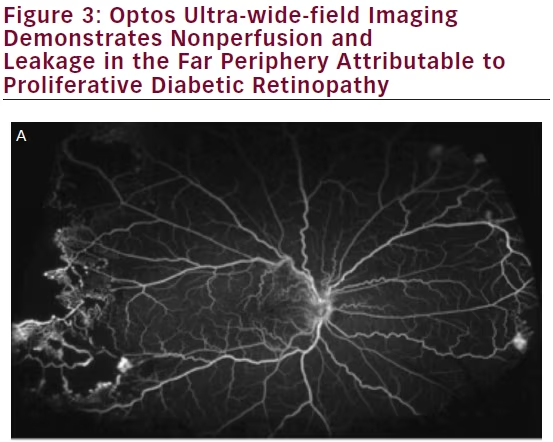
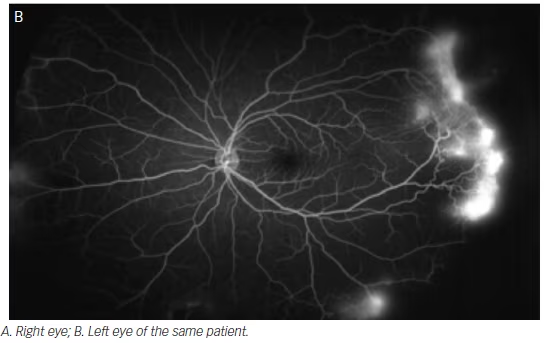
In support of the existing dogma, untreated peripheral nonperfusion and late vascular leakage as detected on ultra-wide-field FA have been found to be associated with neovascularization in diabetic retinopathy.12
The degree of peripheral nonperfusion has also been linked to increased diabetic macular edema (DME) in treatment-naïve patients with diabetic retinopathy,13 as well as more recalcitrant DME, as evidenced by a greater number of macular photocoagulation treatments and less reduction in central macular thickness on optical coherence tomography (OCT).14 Sim et al. did not observe this relationship between peripheral ischemia and central macular thickness, but the authors postulate this may have been due to the higher number of patients with macular ischemia included in their study.15 They did note a relationship between capillary nonperfusion in the peripheral retina and in the central macula, based on the size of the foveal avascular zone. These findings will require further study and validation but, given their variability, may suggest that not all regions of peripheral nonperfusion contribute equally to DME.
Given that neither the degree of retinal nonperfusion nor perivascular leakage was used as a criterion in the ETDRS or Diabetic Retinopathy Study, these markers of disease pathology as seen on wide-field imaging may play an important role in our future management of diabetic retinopathy and DME. Given the potential morbidities associated with panretinal photocoagulation (loss of peripheral vision, decreased scotopic vision, and macular edema), there is a growing interest in performing targeted retinal photocoagulation (TRP) in areas of capillary nonperfusion as visualized on wide-field imaging.16–18 Muqit et al. reported successful regression of neovascularization without induction of macular edema after TRP in treatment-naïve proliferative diabetic retinopathy when compared with traditional pan-retinal laser photocoagulation (PRP).16,17
Finally, nonmydriatic ultra-wide-field imaging may be useful for screening diabetic retinopathy via telehealth programs in places with limited access to care.
Retinal Vein Occlusion
Unlike diabetes, retinal venous occlusions have been classified historically into ischemic versus nonischemic based on the degree of retinal nonperfusion on FA. Given our ability to more accurately visualize and measure peripheral capillary nonperfusion on wide-field FA, an ischemic index has been postulated for central retinal vein occlusions.19 The ischemic index is calculated by the amount of nonperfused retina to total visualized retina. It has been shown to correlate directly with the presence of neovascularization, as well as the degree of macular edema and response to antivascular endothelial growth factor (anti-VEGF) treatment.20 While the ischemic index has been helpful in the context of research analysis of wide-field angiograms, it has not found its way into the everyday practice of the typical retinal physician. In addition to visualizing the peripheral retina, ultra-wide-field imaging has been shown to accurately demonstrate macular edema and the foveal avascular zone, as measured against spectral domain OCT.21,22
While cystoid macular edema secondary to retinal vein occlusions is classically treated with anti-VEGF injections, one case report demonstrates that wide-field guided assessment of retinal nonperfusion and corresponding TRP may break the cycle of rebound edema that often occurs when anti-VEGF injections are stopped.23 This supports the theory that peripheral ischemia may drive macular edema in some patients, especially those with refractory macular edema or limited response to anti- VEGF and steroid treatments. Further prospective studies will be necessary to validate whether treatment of these peripheral areas via modalities such as TRP will benefit such patients.
Pediatric Retinal Diseases
Several pediatric diseases have prominent peripheral retinal findings, including ROP, Coats disease, and familial exudative vitreoretinopathy. In particular, imaging with the RetCam has been shown repeatedly to correlate well with clinical exams using binocular indirect ophthalmology (BIO) for treatment-requiring ROP.9,24–41 While these studies had different inclusion criteria making direct comparisons difficult, in general, widefield imaging appears more sensitive with increasing ROP severity, with BIO found to be superior in cases of mild ROP, especially those manifesting in the periphery.42 Noncontact ultra-wide-field oral FAs have also been used successfully in premature infants to evaluate for ROP.43 Overall, wide-field imaging currently serves as an adjunct to rather than a replacement of BIO, given the greater ease of viewing the peripheral retina with the latter. The authors do not recommend using wide-field imaging as a screening device for all patients to look for peripheral retinal pathology. Rather, the device is best used to document pathology that is detected using standard clinical examination techniques.
Wide-field imaging is particularly useful in settings where a trained examiner is not readily available and telemedicine can be used to screen for acute-phase retinopathy. The efficacy of teleretinal screening for ROP using RetCam has been validated in several studies.27–29,35–41,44 Notably, the latest 6-year results for the Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP) telemedicine initiative found that 22 of 1,216 total eyes examined (3.6 %) met criteria for treatment-warranted ROP. Compared with bedside BIO, remote RetCam photographic analysis by an ROP specialist demonstrated remarkable diagnostic accuracy, with a sensitivity of 100 % and specificity of 99.8 %.41 Recently, the multicenter Telemedicine Approaches to Evaluating Acute- Phase Retinopathy of Prematurity (e-ROP) study created a grading system for digital ROP images, where nonphysicians were successfully trained to grade and screen for patients with referral-warranted ROP.45 These findings suggest that telemedicine appears to be a safe, reliable, and cost-effective complement to direct examinations by ROP specialists, with the potential to increase screening and extend treatment to infants with vision-threatening disease.
Hemoglobinopathies
Wide-field imaging aids in diagnosing subclinical sickle cell retinopathy, thereby increasing awareness of this systemic disease and allowing for earlier identification of patients at higher risk of retinopathy.46 A retrospective case series of 12 eyes in six patients compared Optomap FA with standard seven-field photography and found that Optomap captured all findings that were missed in all but one eye by seven fields.47 Similarly, wide-field imaging revealed neovascularization not evident clinically in patients with beta-thalassemia major.48 Such findings suggest that these higher-risk patients may benefit from regular surveillance with wide-field imaging for peripheral vascular abnormalities.
Uveitis
Ancillary investigations are the backbone of diagnosing and managing uveitis involving the intermediate and posterior segments. The superiority of wide-field imaging compared with conventional equivalents has been demonstrated for color fundus photography,49–51 FA,49,50,52,53 and AF,54,55 in posterior uveitis, as well as for FA in intermediate uveitis (see Figure 4).56 A single 200° Optos FA image not only captured a mean 1.5 times the area
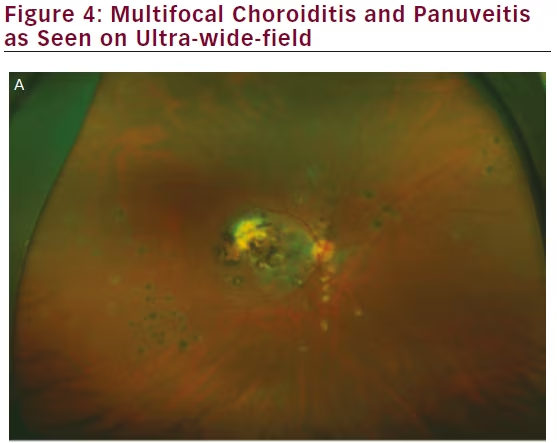
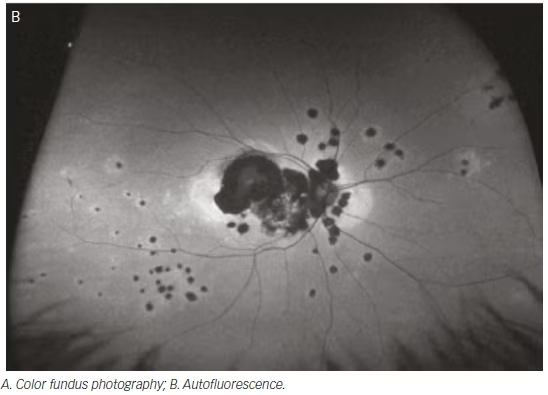
captured by the nine-field montage, but also increased visualization of retinal vascular leakage (median 22.5 mm2 versus 4.8 mm2).50
This improved visualization of the extent of chorioretinal involvement and vasculitis may have direct implications on management. A prospective case series involving 43 patients with noninfectious posterior uveitis determined a change in management in seven of 43 patients (16 %) based on clinical examination and conventional angiography compared with 21 of 43 patients (48 %) based on ultra-wide-field imaging and angiography.57 However, there was no significant difference in disease activity based on the two imaging modalities. Further longitudinal studies will be required to determine whether this increased number of findings provided by widefield imaging and subsequent alteration in the course of management will improve patient outcomes.
Chorioretinal Diseases
Many inherited retinal dystrophies have characteristic patterns of pathology. These are more easily recognized on ultra-wide-field imaging, which allows maximal visualization of the posterior segment in a single image. Yuan et al. retrospectively identified cases of gyrate atrophy, choroideremia, and the carrier state of choroideremia with ultra-wide-field fundus photography and FA.58 Cases of gyrate atrophy demonstrated diffuse confluent chorioretinal
atrophy from the posterior pole to the periphery in contrast with the patchy, irregular pattern of atrophy seen in choroideremia.
In addition, wide-field color fundus photography, FA, and AF are useful for diagnosing and monitoring peripheral choroidal lesions, including choroidal nevi versus melanomas59–61 and choroidal metastases.62
Retinal Detachment
Studies have suggested that wide-field imaging is not sufficient to detect all retinal tears, breaks, and detachments, even exclusive of its inability to image the far anterior retina, and it is unable to fully convey postoperative changes after retinal detachment repair. Thus, while useful as an adjunctive test, widefield imaging does not replace the clinical examination in these cases.
In one study comparing Optos imaging with retinal ophthalmoscopy, Optos detected seven of 10 cases with retinal breaks and holes, with those missed present superiorly and inferiorly, as well as nine of 10 retinal detachments.63 In a retrospective study involving 36 eyes of 34 patients, wide-field more precisely documented the extent of retinal detachments in 13.9 % of cases, but failed to detect retinal holes in the superior and inferior quadrants in 11.1 % and 19.4 % of cases, respectively.64 Retinopexy was not detected superiorly and inferiorly in 19.4 % of cases. Finally, a third study found moderate sensitivity for lesions posterior to the equator and low sensitivity anterior to the equator.64 Specifically, for lesions posterior to the equator, sensitivity overall was 74 %, and 76 % for lesions requiring treatment, whereas for lesions anterior to the equator, sensitivity overall was 45 % and 36 % for treatable lesions. Specificity was 85 %.
While the Optos Optomap covers a larger total retinal surface area on FA, this wider view is pre-eminently temporally and nasally. With a single, nonsteered shot centered on the macula, the Heidelberg Spectralis noncontact ultra-wide-field FA was found to provide greater views of retinal vasculature superiorly and inferiorly.8 Therefore, if a patient has been identified with pathology in a far superior or inferior distribution, the Optos platform may not be the optimal device used to document these findings.
Macular Diseases
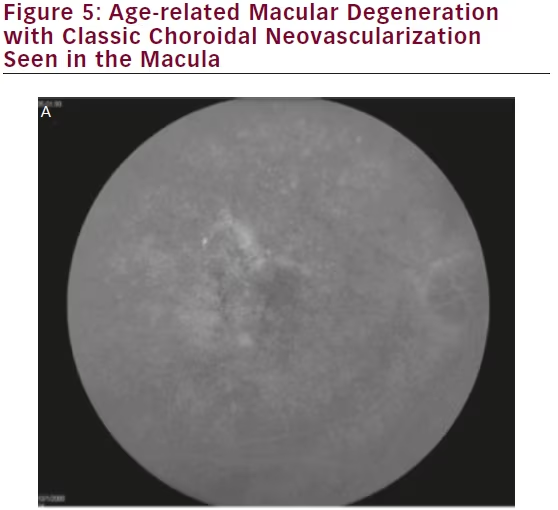
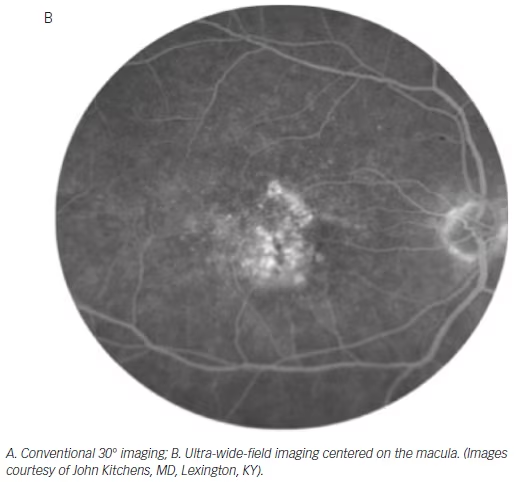
Conventional imaging has historically had the highest resolution for visualizing macular pathology. However, studies have demonstrated that wide-field imaging systems are equally effective in diagnosing macular pathology as standard 45° fields of view (see Figure 5), with one study demonstrating a 96.4 % agreement between conventional and ultra-wide-angle imaging in grading macular diseases, with no clinically significant disagreements.66
Furthermore, there is mounting evidence that central retinal pathologies are often linked directly to peripheral changes. Prior to the era of widefield imaging, our understanding of the relevance of peripheral retinal abnormalities in diseases such as age-related macular degeneration (AMD) were limited by the lack of detailed peripheral imaging studies. Lengyel et al. reviewed 567 random subjects aged 62 or older and found AMD-like changes in 10.1 % of this population in the periphery alone, 13.6 % in the macula alone, and 57.4 % in both the periphery and macula.67 Witmer et al. analyzed 157 eyes in 83 AMD patients using ultra-wide-field AF and found peripheral AF abnormalities in 63.6 % of eyes with AMD versus 35.7 % of control eyes (p=0.049).68 Particularly, granular fluorescent changes and patchy hypo-AF were more common in eyes with advanced AMD, including choroidal neovascularization and geographic atrophy, than those with early AMD or in control eyes. These findings suggest that new grading structures or phenotypic characterizations may be established for AMD in the future based on ultra-wide-field imaging.
Future Directions and Conclusions
With continuing advances in technology and increasing clinical applications, the use of wide-field imaging is on the rise and will likely soon become the standard of care in fundus imaging. What was popularized as an endeavor for examining pediatric retinas is rapidly becoming ubiquitous for examining myriad posterior segment pathologies in a wide patient population. A growing number of retinal diseases are increasingly recognized as demonstrating changes in the peripheral retina not previously seen before the advent of wide-field imaging, and these changes are often linked to earlier manifestations of disease. These findings are prompting more timely diagnosis and may be used to guide specific treatments.
Further studies will be necessary to identify unifying grading systems for various diseases using wide-field imaging, and it will be important to elucidate the implications of these new findings for disease prognosis and management. Other future considerations for wide-field imaging include further integration of other imaging modalities, including the possibility of a wide-field OCT, as well as a smaller form factor. In spite of the significant strides made in wide-field imaging, it remains limited in capturing the anterior retina, where binocular indirect ophthalmoscopy remains the gold standard. Thus, the ideal wide-field imaging system of the future will be able to image the retina accurately from ora to ora without significant distortions and artifacts, while maintaining a high degree of sensitivity and specificity for even the subtlest retinal pathology, with multiple imaging modalities available on the same platform.







