Ultrasound cycloplasty (UCP) is a technique that utilises high-intensity focused ultrasound (HIFU) to provide controlled and smooth thermal coagulation of the ciliary body; it has been shown to be effective at reducing intraocular pressure (IOP) in patients with advanced or refractory glaucoma.1–5
Recently, the results of a 12-month, prospective, multicentre European clinical study of UCP in surgery-naive patients with glaucoma (NCT02789293)6 have been made available and show promising efficacy and safety data, consistent with earlier studies.1–5 Given this, it is time to discuss and reconsider the positioning of UCP in the clinical care pathway for glaucoma, potentially incorporating it as a treatment option for earlier stage/non-refractory disease.
Here we present new clinical study data on the use of UCP in patients with glaucoma, including those from the European clinical study in surgery-naive patients, and discuss the implications of these findings for clinical practice.
Ultrasound Cycloplasty – What is the Mechanism of Action?
Presented by: Phillippe Denis
Croix-Rousse University Hospital, Lyon, France
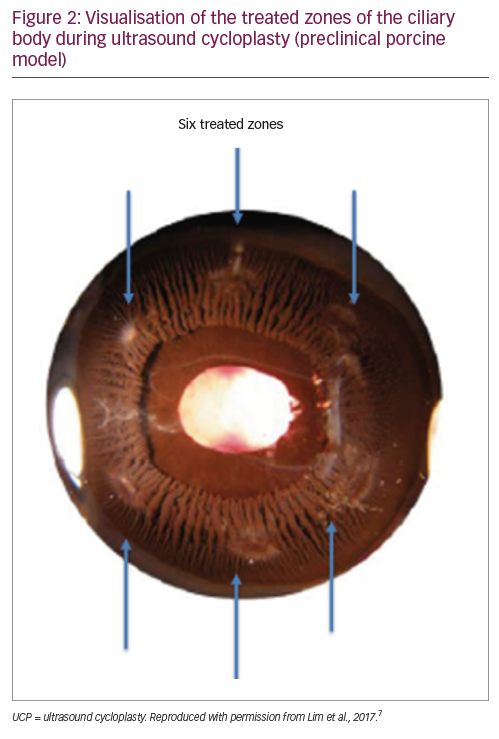
While the rationale behind UCP is established (removal of the ciliary body epithelial cells to reduce aqueous humour production), the exact mechanism through which the procedure achieves a reduction in IOP has, until recently, not been fully characterised. UCP is anon-invasive technique that generates smooth, focussed and controlled (via stable and progressive transfer of energy) thermal coagulation of the epithelial cells of the ciliary body (Figure 1), preserving theblood–aqueous humour barrier and avoiding tissue explosions that are observed with more aggressive cyclodestructive techniques such as diode laser transscleral cyclophotocoagulation.7,8 This more gentle and progressive approach may also explain why, in some cases, patients may require a second UCP treatment to reach the desired IOP.9UCP also utilises six active transducers to treat six separate areas of the ciliary body, covering approximately 40% of the total surface area, thereby limiting the risk of hypotony (Figure 2).7 Indeed, no phthisis bulbi or permanent hypotony have been observed with the procedure in published studies.2,3,5,9,10

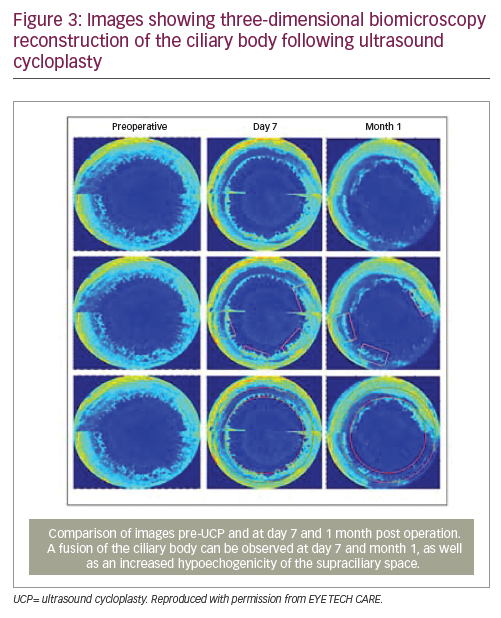
Recent studies using ultrasound biomicroscopy to visualise the ciliary processes and sclera have confirmed that the main mechanism of action of UCP is the reduction of aqueous humour production via partial coagulation of the ciliary body, resulting in atrophy of the ciliary processes (Figure 3).11 A complementary mechanism of action might be increased aqueous humour outflow due to a widening of the uveoscleral pathway, occurring at least temporarily.7,11
In conclusion, UCP is a non-invasive procedure that acts via coagulation of the ciliary body and provides the possibility to conduct multiple treatments if necessary.9
Ultrasound Cycloplasty European Study Results – 1-year Follow up in Surgery-naive Patients with Glaucoma
Presented by: Ingeborg Stalmans
University Hospitals UZ Leuven, Leuven, Belgium
Based on available clinical study data, UCP is recommended for the treatment of IOP in adults (aged ≥18 years) with refractory primary open angle glaucoma or primary angle closure glaucoma.2 It can also be used following failed filtration surgery.2,3,5,12 UCP is not recommended in patients with an IOP of <21 mmHg due to an increased risk of hypotony. As the average IOP decrease is around 30–40%, patients with a very high IOP may require a second or third UCP treatment to reach the target pressure.9 UCP can also be considered in patients with secondary glaucoma (e.g., pigmentary, pseudoexfoliative or neovascular glaucoma), though the response rates have been shown to be lower in these indications compared with primary open angle glaucoma (~50% versus 65–70%).2–4
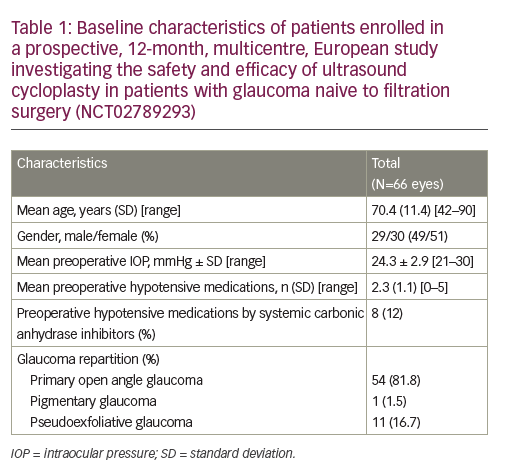
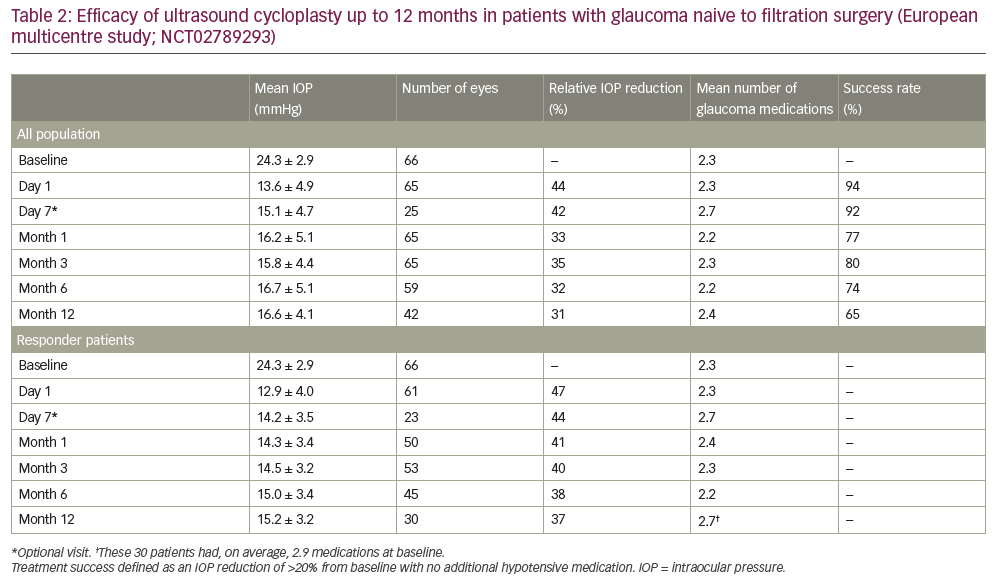
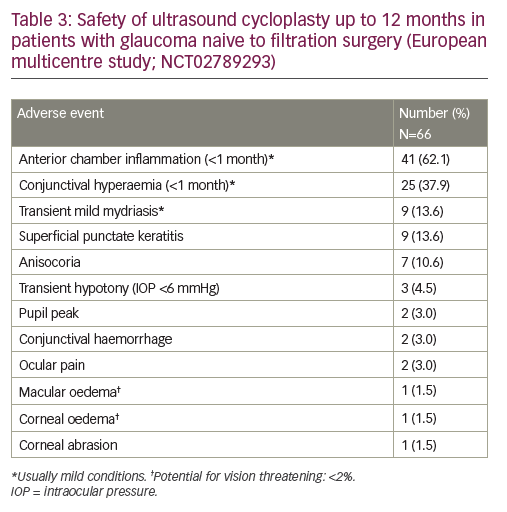
In addition, UCP can be considered for the reduction of IOP in patients with non-refractory glaucoma.2,13 Here we present the results of a prospective, 12-month, multicentre, European study conducted in four university hospital centres in Belgium, Portugal, Israel and Italy to investigate the safety and efficacy of UCP in patients with glaucoma naive to filtration surgery (NCT02789293).6Overall, 59 patients were enrolled in the study and UCP was performed in 66 eyes. Baseline characteristics are summarised in Table 1; the large majority of patients had primary open angle glaucoma(n = 54 [81.8%]), the mean baseline IOP was 24.3 ± 2.9 mmHg, and the mean number of preoperative hypotensive medications used was2.3 ± 1.1 per patient. Following a single UCP treatment, patients were assessed at day 1, day 7 and at 1, 3, 6 and 12 months.After 12 months, the mean IOP reduction for all patients was 31% (mean IOP: 16.6 ± 4.1 mmHg), the response rate was 65% (defined as an IOP reduction of >20% from baseline with no additional hypotensive medication; Table 2) and the mean IOP reduction for responders was 37% (mean IOP: 15.2 ± 3.2 mmHg). In addition, 76% and 62% of eyes had an IOP of <21 mmHg and <18 mmHg, respectively, at 12 months. Throughout the study the mean number of hypotensive medications used remained broadly consistent (range: 2.2–2.7 per patient).
Overall, the safety profile of the UCP procedure was favourable. Post-operative adverse events were predominantly mild and transient, most commonly anterior chamber inflammation (occurring <1 month after the procedure; n=41 [62.1%]), conjunctival hyperaemia (<1 month; n=25 [37.9%]), transient mild mydriasis (n=9 [13.6%]), and superficial punctate keratitis (n=9 [13.6%]; Table 3). There were few vision-threatening complications, which were largely transient; transient hypotony occurred in three patients (4.5%) and macular oedema in one (1.5%). Visual acuity was maintained at both 6 months and 12 months post operation, with no significant differencesin best-corrected visual acuity (BCVA) values compared with baseline (6 months versus baseline: 0.404 versus 0.328, p=0.136; 12 months versus baseline: 0.301 versus 0.220, p=0.379), and no major BCVA change (a decrease of >1 line) in 86% of patients at 12 months.
Recently, other clinical studies have also been published showing 12-month data for the use of UCP in the treatment of glaucoma.9,14 Consistent with the results presented here, these studies show that UCP has a good efficacy and safety profile, with responder rates up to 75% at 12 months and an average IOP reduction of 30–35% from baseline.9,14 While UCP was initially considered only in patients with refractory or advanced glaucoma, the clinical data now available (especially in surgery-naive patients) indicate that UCP may be a viable option for early-stage patients, and could be included as part of personalised medicine strategies in the future.
Complications – What is the Reality?
Presented by: Luís Abegão Pinto
Santa Maria University Hospital, Lisbon, Portugal
UCP has established efficacy, with up to 75% of patients responding to treatment after 12 months with an average IOP lowering effect of 30–35%.9,14 In addition, UCP provides the option of stitchless surgery, and thereby a quick overall recovery (typically ~1-day of mild post-operative discomfort) for the patient. The UCP procedure itself is non invasive, reproducible and simple, with a short and user-friendly learning curve, and can be completed within 10–15 minutes (with ~3 minutes of actual operating time). These benefits are reflected in the growing interest in the procedure. As such, establishing the safety profile of UCP and the risk factors for potential complications is of key importance, providing both surgeons and patients alike with greater insights into what to expect from UCP and how and when it is best to be performed.
Results from the prospective, 12-month, multicentre, European study mentioned above (NCT02789293) show that the majority of post-operative adverse events were mild and transient conditions (Table 3). Most were related to inflammation of the anterior chamber (n=41 [62.1%], which was to be expected as UCP involves some heating of the ciliary body. From the patient’s perspective, on day 1 post operation there was usually some tingling reported, but no pain or major discomfort. There was a small risk of more serious complications that are potentiallyvision-threatening, such as macular or corneal oedema, but these were rare and of a similar incidence (n=1 [1.5%]) to the levels accepted for other ocular surgeries (e.g. cataract surgery).15 Importantly, there was no phthisis bulbi (or ciliary shutdown) reported following UCP, which is consistent with published data from other clinical studies.2,3,5,10
In relation to post-operative pupil events (e.g., transient mild mydriasis, anisocoria or pupil peak), while these rarely lead to complaints from the patient, there may be specific risk factors that could predict the likelihood of these events occurring. Potential causes might include: (i) incorrect centration or probe sizing; (ii) Urretz Zavalia Syndrome (as seen during penetrating keratoplasty surgery);16 (iii) perioperative mydriasis; or (iv) an intraoperative increase in IOP.
To investigate potential ocular risk factors and assess prophylactic options, a pupillometry sub-study was conducted within the 12-month European study (NCT02789293). In patients enrolled at the Leuven (Belgium) or Santa Maria (Portugal) University Hospitals, pupil dynamics were assessed using an infra-red pupillometer (Plusoptix®S04; Plusoptix, Georgia, USA) at various post-operative timepoints (day 1, 1 month, 3 months and 6 months). Exclusion criteria for the sub-study included previous trabeculectomy, uveitis, diabetes or another autonomic nervous system disorder, severe ametropia or an inability to fixate on the target during pupillometry.
Of the 16 patients eligible for the sub-study, 10 (62.5%) were female, the mean age was 69 ± 11 years, the mean BCVA was 0.16 ± 3.0 logMar, the mean IOP was 23.6 ± 3.0 mmHg, and the mean number of hypotensive drugs used was 2.4 ± 1.3 per eye. During each pupillometry assessment, the vertical and horizontal diameter of the pupil under mesoscopic (1 cd/m2) and scotopic (0.1 cd/m2) conditions (using a pupil change threshold of 20%) were assessed. After 1 month, pupillometry data showed that there was a significant difference in light–dark pupil response compared with baseline (pupil diameter difference: 0.03 ± 0.34 versus 0.38 ± 0.30 mm, respectively; p=0.01), indicating hypomobility of the iris. However, after 3 months and 6 months the iris mobility had returned to near-baseline levels(pupil diameter difference: 0.28 ± 0.49 and 0.23 ± 0.41 mm, respectively; both p>0.1), suggesting the effect to be transient. Visual acuity appeared unrelated to iris mobility, with BCVA values similar to baseline through months 1–6 (range: 0.17–0.23 logMar; all p>0.05). Analysis of thevertical/horizontal ratios of the pupils showed that they were round, indicating that there was no corectopia due to errors in centration or probe sizing. Also, neither univariate nor multivariate analysis of any of the ocular parameters studied was associated with an increased chance of an altered pupil response. As such, it is not currently possible to select patients based on ocular characteristics to provide pupil-effect-free UCP procedures.
In conclusion, UCP is an effective IOP-lowering procedure with a favourable safety profile and few vision-threatening complications. In some cases, it may induce a transient mild mydriasis immediately following the procedure, but this does not substantially impact visual acuity and the condition will typically resolve without additional treatment in a few months.
Multiple Treatments – Why, When and How to Re-treat?
Presented by: Simonetta Morselli and Alessandra De Gregorio
San Bassiano Hospital, Bassano del Grappa, Italy
While clinical studies have shown that UCP with HIFU is an effective technique,2–5,9,14 one treatment is sometimes not sufficient to reach the target IOP, particularly in patients who may have a very high IOP at baseline.9 Also, some patients who do show an initial response to treatment may then experience a relapse where the IOP returns back to pre-UCP levels.9 In these cases, it may be that smooth thermal coagulation of the ciliary body via UCP has allowed subsequent regeneration of the epithelium, re-starting the production of aqueous humour. It was hypothesised that additional UCP procedures may improve the effectiveness of treatment in these patients.9
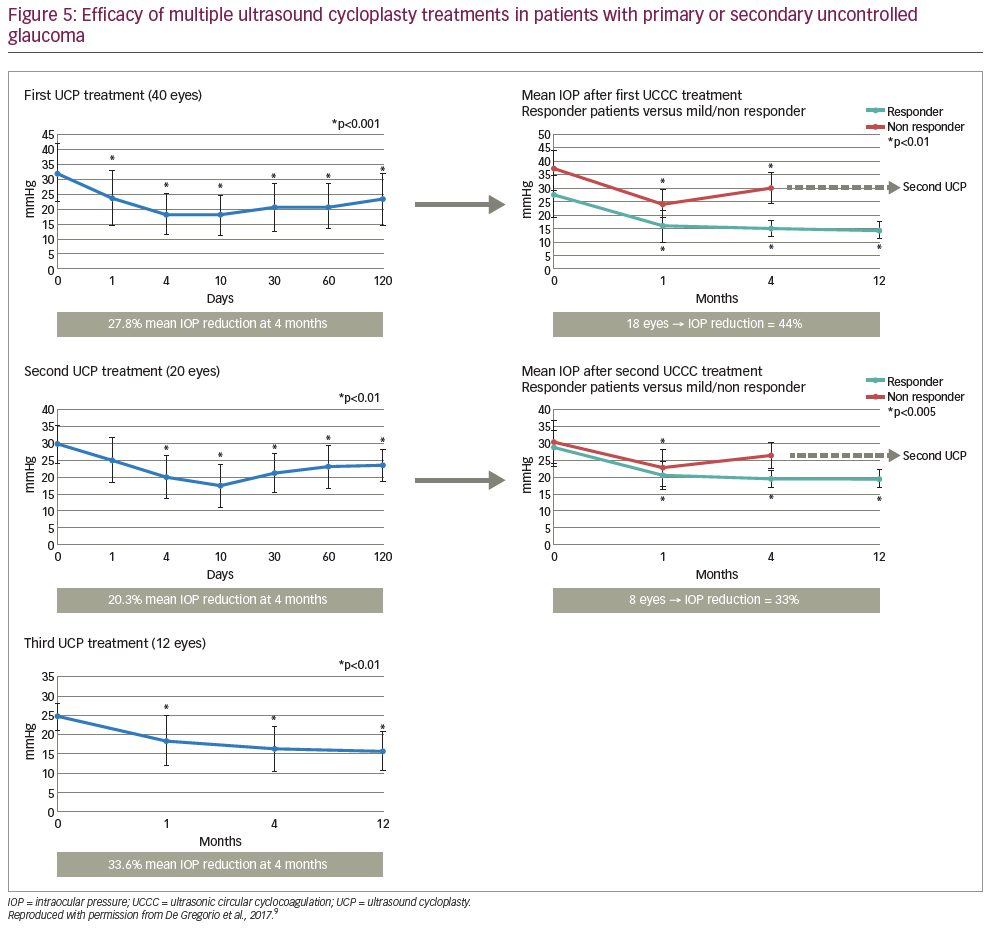
Here we present the results of a prospective, non-comparative, case series study investigating the safety and efficacy of multiple UCP treatments in patients with primary or secondary uncontrolled glaucoma.9 In total, 40 patients were enrolled in the study, the mean age was 71.8 ± 13.6 years, 20 (50%) were female, 17 (42.5%) had received previous filtering surgery, the majority had primary open angle glaucoma (n=14 [35.0%]), exfoliative glaucoma (n=12 [30.0%]) or neovascular glaucoma (n=8 [20.0%]), and the mean preoperative IOP at baseline was32.5 ± 9.9 mmHg.9 UCP was performed on 40 eyes; if the first treatment was successful (defined as an IOP of ≤21 mmHg 4 months after the procedure), then no further UCP procedures were performed. If the IOP was >21 mmHg after 4 months, then a second UCP procedure was performed without rotating the probe (as has been carried out in a previous 12-month prospective study).3,9 This was to ensure the same area was targeted as per the first procedure and account for any potential regeneration of the epithelium, even if partial, on the ciliary body.9 The same process was then repeated if a third UCP procedure was required after another 4 months (Figure 4).
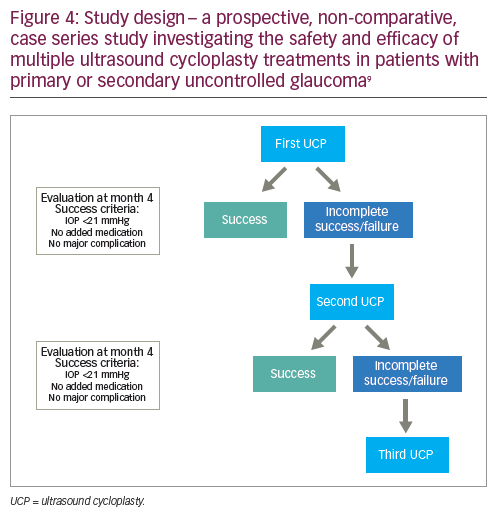
Four months after the first UCP procedure, the mean IOP reduction from baseline was 27.8% (Figure 5). At this stage, 22 of the treated eyes had not achieved complete success and a second treatment was performed in 20 eyes (one patient refused further treatment and one underwent trabeculectomy surgery).9 Four months after the second UCP procedure, the mean IOP reduction was 20.3% from preoperative values and 34.7% from baseline; a third treatment was performed in 12 eyes. Four months after the third and final UCP treatment, the mean IOP reduction was 34.0% from preoperative values and 52.6% from baseline. Overall, multiple treatments increased the efficacy of UCP treatment, with 34/40 (85%) patients achieving an IOP of <21 mmHg with no added medication 12 months after the last procedure. In addition, the mean number of hypotensive therapy classes required by patients was significantly decreased after 12 months compared with baseline(2.4 ± 1.3 versus 3.6 ± 0.7; p<0.01).9
Post-operative adverse events were predominantly mild and transient, most commonly conjunctival hyperaemia (n=40 [100%]), superficial punctate keratitis (n=18 [45%]) and sub-conjunctival haemorrhage (n=12 [30%]). The mean visual acuity remained similar to baseline.9 In two cases a two-line visual loss was reported, but neither was related to the UCP procedure (one due to cataract evolution, one due to glaucoma progression). Two corneal ulcers were also reported, but these were rapidly resolved with additional treatment and were judged to be due to pre-existing corneal surface disease. There were no vision-threatening complications during or after any of the UCP procedures (e.g., no permanent phthisis bulbi or hypotony).9
In conclusion, multiple UCP treatments increase the overall efficacy of the procedure, without increasing the risk of complications.
Discussion and Conclusions
Evidence from recent clinical studies has shown that UCP is an effective treatment for glaucoma, providing 30–35% reductions in IOP for up to 12 months in 65–85% of cases.9,14 Adverse events are typically mild and transient (usually minor inflammation), with vision-threatening complications occurring rarely (in <2% of procedures). Because UCP is a non-invasive and less destructive method of reducing IOP compared with other cyclo-destructive techniques (e.g. diode laser transscleral cyclophotocoagulation), providing smooth, controlled coagulation of the ciliary body, multiple treatments can be performed on the same patient to increase the likelihood of treatment success without increasing the risk of complications.
The data presented here open the perspective for changes in the glaucoma clinical care pathway. While UCP was initially established for the treatment of refractory/advanced glaucoma, the promising efficacy and favourable safety profile observed in 12-month studies in surgery-naive patients with glaucoma (NCT02789293)2,13 suggests that UCP may also be a viable therapeutic option in patients with moderate-stage/non-refractory glaucoma. Future studies may further refine the role of UCP, which could form part of personalised medicine strategies to optimise patient outcomes.
|
“UCP uses focussed ultrasound resulting in a localised treatment effect with very little collateral damage (compared with other techniques)” – Simonetta Morselli “Initially, UCP was used only for refractory glaucoma, but it can also be used in surgery-naive patients” – Philippe Denis “Overall, I think UCP is meant for mild-to-moderate glaucoma in patients who haven’t had previous surgery” – Luís Abegão Pinto “Thank you for confirming that the study results (of NCT02789293) are consistent with your clinical experience and for opening the future perspective – a key goal is personalising UCP treatment for individual patients” – Ingeborg Stalmans |







