Diabetic retinopathy (DR) is the most common cause of moderate-tosevere visual impairment among working class adults.1 Diabetic macular oedema (DMO), a common manifestation of DR, causes central vision loss,2 and is a consequence of vascular inner blood–retinal barrier breakdown.3 Global prevalence of DR among diabetic individuals is approximately 35 %, with DMO present in 6.8 %.4 DMO is difficult to manage due to its chronicity. Historically, the standard of care was laser therapy; however, a large number of patients continued to lose vision,1,5 with significant vision recovery in only 15 % of treated patients.11 In particular, patients presenting with diffuse DMO, where causal vascular abnormalities are not restricted to discrete foci, are likely to be refractory to laser therapy.6 Given the proportion of patients with DMO refractory to laser therapy, new therapies providing sustained benefit are needed.7
Alternative DMO treatments are evolving rapidly, with a number demonstrating promise in preventing visual acuity (VA) deterioration and improving vision.1,2 One such treatment is fluocinolone acetonide (FAc) (0.2 μg/day, ILUVIEN®),8 a non-biodegradable, intravitreal (IV) corticosteroid implant,9 which releases daily sustained, low-dose FAc for 3 years.10 The Fluocinolone Acetonide in Diabetic Macular Edema (FAME) study compared 0.2 μg/day FAc with sham injections in patients with DMO, a baseline best corrected VA (BCVA) in Early Treatment Diabetic Retinopathy Study (ETDRS) letter score between 19–68 (20/50–20/400), central foveal thickness (CFT) ≥250 μm, and ≥1 prior focal laser treatment.11 Patients receiving non-protocol treatments were included. Patients were eligible for rescue laser after 6 weeks or retreatment with assigned therapy after 1 year.
Three-year treatment with 0.2 μg/day FAc improved BCVA in 34.0 % of chronic (≥3 years median duration) DMO patients compared with 13.4 % sham-treated patients, with mean changes in BCVA score of 7.6 and 1.8 letters, respectively.7 CFT rapidly and substantially decreased from baseline in both FAc- and sham-treated chronic DMO patients.7 Although at 36 months FAc implant patients needed cataract surgery more frequently than those patients who received sham injection, their post-surgery visual benefit was similar to subjects pseudophakic at baseline. Surgery to manage elevated intraocular pressure (IOP) was also more frequent for FAc-treated patients compared with sham (4.8 % versus 0.5 %).7
Report of a Case
A retired, 62-year-old male patient with type 2 diabetes mellitus since 1992 (insulin dependent since 2002) was first treated for DMO in 2004, for which he received left eye (LE) laser photocoagulation and triamcinolone injection when his BCVA was 0.2 for his LE and 0.8 for his right eye (RE). Lost to follow-up until 2011, BCVA had deteriorated to 0.2 in both eyes. From 2011, the patient’s response to therapy was evaluated using BCVA and CFT measured using spectral optical coherence tomography (OCT). IV ranibizumab injection in 2011 gave no benefit for LE BCVA and given the lack of response to therapy and the long history of DMO, no further treatment was administered to the LE at this time.
Three separate, monthly IV ranibizumab injections in the RE, administered between September and November 2011, did not improve BCVA and focal laser photocoagulation was provided: IOP was 16 mmHg. A 0.7 mg dexamethasone implant in December 2011 produced shortterm CFT decrease (442 μm to 347 μm) without improving BCVA (see Figures 1A and B). Six further IV ranibizumab injections over 8 months had no effect on visual outcomes, with CFT worsening (347 to 488 μm; Figure 1B) and IOP increasing (22 mmHg). Following IV ranibizumab injection combined with 0.7 mg dexamethasone implant in October 2012, CFT decreased to 223 μm and BCVA improved from 0.1 to 0.2, lasting 4 months. Two further ranibizumab injections gave no visual improvement. After phacoemulsification in April 2013, and one further ranibizumab injection in May, BCVA (0.25 to 0.2) and CFT (350 to 506 μm) worsened. IOP was 19 mmHg.
In July 2013 the patient had a 0.2 μg/day FAc implant inserted. Within 3 weeks, OCT indicated complete DMO regression that was not achieved with any of the previous IV therapies, resulting in CFT decreasing by 58 % after 10 weeks (548 μm to 232 μm; Figure 1B and Figure 2). These improvements were still apparent 6 months following 0.2 μg/day FAc implantation, with a concurrent increase in BCVA to 0.3 at its highest point (a clinically meaningful change from the previous BCVA of 0.2). In January 2014, OCT showed DMO with a progression of CFT (289 μm). Bevacizumab was injected twice, 3 weeks apart in February 2014, leading to regression of DMO (CFT 282 μm; BCVA 0.25). In July 2014, CFT was seen to be further reduced to 214 μm, and another bevacizumab injection was administered. The patient reported greater vision-related quality of life and the ability to perform daily routines without problems, with no complaints regarding 0.2 μg/day FAc implant therapy.
Management of Intraocular Pressure
Following 0.2 μg/day FAc implantation, gradual elevation in IOP was observed over the first 2 months (from 16 mmHg to 28 mmHg). This was resolved with daily dorzolamide/timolol eye drops (21 mmHg at 6 months). In February 2014, tafluoprost eye drops were prescribed in addition to further reduce the IOP. The daily application of eye drops was not a concern for the patient and did not interfere with his quality of life.
Comments
Randomized clinical trials indicate intraocular vascular endothelial growth factor (VEGF) inhibition provides significant BCVA and CFT improvements in patients with DMO.12 While this is a widely used therapeutic strategy in patients refractory to laser therapy, other studies have shown that anti-VEGF therapy alone is not effective in all patients with DMO,13 as shown in this case.
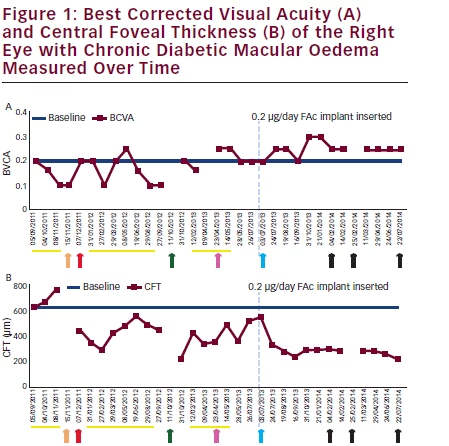
Therapies previous to 0.2 μg/day fluocinolone acetonide (FAc) implant insertion demonstrated, mild, short-term best corrected visual acuity (BCVA) and central foveal thickness (CFT) improvements. 12 months following FAc implant, CFT remains below 300 μm and BCVA is 0.25. Arrow key: orange = laser photocoagulation; red = dexamethasone implant; green = ranibizumab plus dexamethasone implant; pink = phacoemulsification; blue = FAc implant (highlighted by vertical dash blue line); black = bevacizumab. Yellow line = ranibizumab intravitreal injection.
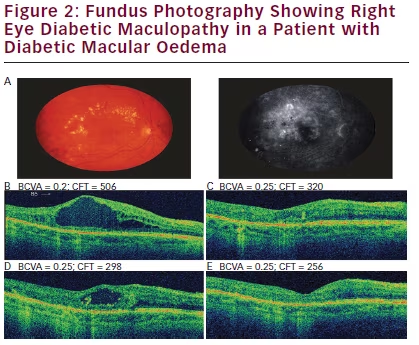
A. Optical coherence tomography (OCT) scan of the right eye (RE) before treatment with 0.2 μg/day fluocinolone acetonide (FAc) implant; best corrected visual acuity (BCVA) 0.2; central foveal thickness (CFT) 506 μm. B. 3 weeks after 0.2 μg/day FAc: BCVA 0.25; CFT 320 μm. C. 7 months after 0.2 μg/day FAc: BCVA 0.25; CFT 298 μm. D. 12 months after 0.2 μg/day FAc: BCVA 0.25; CFT 256 μm. E. Little long-term improvement was seen in foveal thickness from the time initial RE treatment began to just before 0.2 μg/day FAc implantation. Following 0.2 μg/day FAc, CFT improved and stayed below 300 μm (see Figure 1).
The Fluocinolone Acetonide for Macular Edema (FAME) study demonstrated a single IV injection FAc implant can provide significant, long-term BCVA and CFT improvements in chronic DMO patients.7,14 In this case study, a single 0.2 μg/day FAc implant improved BCVA and CFT when the patient was insufficiently responsive to all previous treatments, including ranibizumab, laser photocoagulation and dexamethasone implant. However, dexamethasone implant provides release of corticosteroid over a shorter timeframe compared with FAc implant,15 possibly accounting for the relatively short duration of CFT decrease and lack of VA response observed when administered.
FAc implant gave marked improvements of BCVA and with complete regression of MO after 3 weeks, consistent with the FAME study,7 which were still apparent after 6 months. Additional anti-VEGF therapy in February 2014 appeared to increase these benefits, reflecting the multifactorial nature of DMO.16 IOP was elevated with 0.2 μg/day FAc, but was effectively managed by twice-daily dorzolamide/timolol and oncedaily tafluprost eye drops. Given the lack of response to other currently available therapies, and the improvement in vision, the benefit–risk ratio following the 0.2 μg/day FAc implantation is positive for this patient and highlights the importance of early intervention when a patient is responding insufficiently, or not responding, to anti-VEGF therapy. The current outcomes also suggest that to achieve functional benefits from DMO resolution (e.g. BCVA of 0.5 to permit holding a standard driving licence), earlier treatment should be considered.
This case illustrates how 0.2 μg/day FAc implant, with its long-term, low-dose corticosteroid, can provide effective long-term therapy (up to 36 months with one injection) with manageable side effects and an improvement in quality of life and general daily living. It is rewarding to note that our patient reported greater vision-related quality of life and the ability to perform daily routines without problems following 0.2 μg/day FAc implant. In patients with chronic DMO, the relative functional benefits and manageable side-effect profile of 0.2 mg/day FAc implant support its use in patients that are insufficiently responsive to available therapies.
To summarise, 0.2 μg/day FAc implant could have been considered earlier in the treatment of DMO and once the patient had shown limited or no response to anti-VEGF therapy. The current case shows CFT improved following treatment with dexamethasone. If ILUVIEN had been licensed at the time, it may have been considered at this stage to help stabilise CFT and BCVA.







