Retinopathy of prematurity (ROP) is a vasoproliferative disease of preterm infants that continues to be a major cause of preventable blindness worldwide despite concerted efforts to reduce its frequency.1 In developed countries, advances in perinatal care, a reduction in mortality rates of preterm infants, and increased surveillance for ROP has had unintended consequences and has resulted in nearly a 10-fold increase in ROP cases worldwide in the past 20 years.2–4 In the United States, nearly 16% of premature infants hospitalized for more than 28 days develop ROP, and ROP is responsible for 6–20% of childhood blindness in the country.1,5,6 As a result, the National Eye Institute estimates that approximately 14,000 neonates a year in the United States develop ROP, and of these approximately 400–600 become completely blind.7 In 2010, a meta-analysis estimated 184,700 new cases of ROP worldwide of which 20,000 babies became blind, and an additional 12,300 developed mild to moderate visual impairment due to the disease.8
Besides the obvious toll on patients and families, blindness acquired in infancy has a significant economic impact as well. In Peru, early treatment of ROP to prevent neonatal blindness resulted in nearly a $200,000 cost savings to the society (in US dollars).9 The authors of the study further estimated that this would result in $516 million cost savings across a generation in Peru.9 This finding has been reiterated in the developed world as well.10 Consequently, ROP is a leading cause of childhood blindness worldwide with a significant economic impact.11
Many attempts have been made to quell ROP due to its consequences both for the patient and community. Oxygen monitoring and regulation to reduce high oxygenation has had a noticeable effect on reducing the risk of developing ROP in preterm infants.12 Furthermore, teleretinal imaging has shown promise in detecting ROP of any stage, with sensitivities of 76–97% and specificities of 89–100% in a meta-analysis.13 While this will presumably make ROP classification more accessible to areas of the world lacking trained specialists, it is unclear what impact it will have on the disease treatment and outcomes. One burden is the concern that there are insufficient numbers of trained ophthalmologists to treat infants with severe ROP.14 Despite these recent advances in surveillance and treatment, the disease remains an important cause of childhood blindness worldwide and likely will be for the foreseeable future.
Role of vascular endothelial growth factor in angiogenesis
Vascular endothelial growth factor (VEGF) is an important growth factor in angiogenesis and lymphangiogensis that promotes vessel sprouting, endothelial cell growth, survival, and homeostasis.15,16 VEGF consists of a family of six subtypes that signal through activation of three distinct receptors that have been discussed extensively elsewhere (Figure 1).17–19 Dysregulation of VEGF-A and its activation of VEGF receptor (VEGFR)-1 and -2 are believed to be an underlying mechanism in ROP, and for simplicity, VEGF-A will subsequently be referred to as VEGF throughout
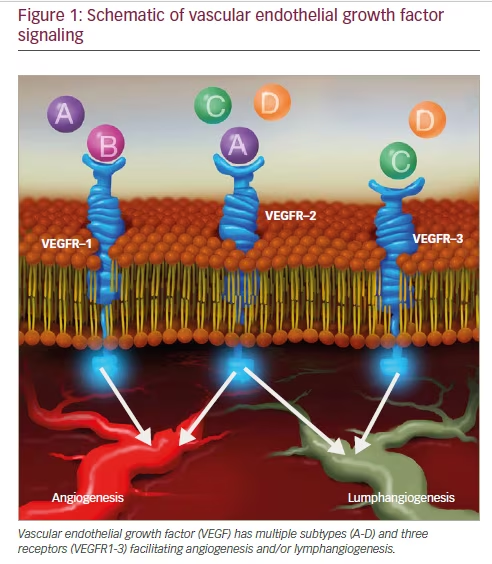
this manuscript.20 VEGF production is highly regulated by local host oxygen concentrations and tissue demands. VEGF is regulated by hypoxiainducible factor-1, a transcription factor that accumulates in hypoxic conditions due to a concurrent decrease in proteolytic degradation.21–24 This has been clearly shown to occur both temporally as well spatially in ischemic mouse retinas.25 Thus, VEGF is highly regulated by oxygen conditions at the cellular and molecular level in diseased tissue.
However, the role of VEGF in endothelial cell survival requires other factors to induce its full angiogenic effect. A non-oxygen-regulated growth factor, insulin-like growth factor (IGF)-1, is another such factor that is able to activate Akt (Protein Kinase B).26–28 Akt is a serine/threonine kinase required to promote cell survival by way of phosphoinositide 3-kinase (PI3K) and subsequent activation of cell survival signals and, thus, regulate VEGF signaling to change physiologic to pathologic angiogenesis.29–32 In fact, low levels of IGF-1 suppress VEGF-survival signaling, and altered concentrations of IGF-1 result in abnormal vascularization of the mouse and presumably human retina in diseases such as ROP.30,33–35 Consequently, the relative concentrations of VEGF and IGF-1 are some of many defining components of the two “phases” discussed below.36 Unfortunately, neither VEGF nor IGF-1 serum concentrations has been shown to have a predictive role in identifying neonates that will develop progressive ROP.37
Pathophysiology of retinopathy of prematurity ROP is classified by zone, stage, and the presence of plus disease.38,39 Experimentally, ROP has been studied using models of oxygen-induced retinopathy (OIR).40 The initial OIR studies were done in the cat in the 1950’s when oxygen levels were not regulated or monitored and when premature infants were larger and of older gestational ages. From those
early studies, two phases were used to describe OIR: an early hyperoxiainduced vaso-obliterative phase (Phase I) followed by a later hypoxiainduced vaso-proliferative phase (Phase II). The phases are helpful to model discrete time points in studying the pathogenesis of OIR, but the phases rarely translate to changes in the individual human preterm infant retina. Also, use of high oxygen is avoided in the US and countries with the technology to regulate and monitor oxygen. OIR models that are representative of human ROP are used to understand pathophysiology.40,41
Staging of retinopathy of prematurity
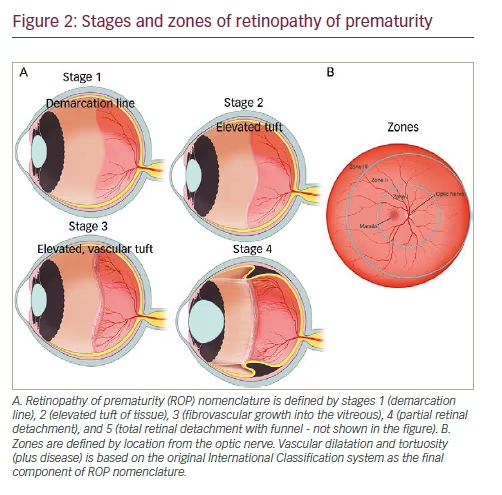
In the mid-1980s, the International Classification of Retinopathy of Prematurity was published to guide diagnosis and treatment of ROP and was then updated in the early 2000s as more information on ROP became available (Figure 2).38,39,42 This classification system has since become the basis of multiple, multicenter, randomized controlled trials as it standardized ROP grading. These subsequent trials have since defined prevention, surveillance, and treatment of ROP. The nomenclature was defined by zones and stages of location, extent of stage and the presence of vascular dilatation and tortuosity (plus disease) in the International Classification system.42 Additionally, the Early Treatment for Retinopathy of Prematurity (ET-ROP) study, has changed the nomenclature of severe ROP to type 1 versus type 2 ROP from analysis of retrospective data from the ET-ROP study.43 Type 1 ROP includes eyes with zone I and any stage ROP with plus disease, zone I stage 3 without plus disease, or zone II with stage 2 or 3 and plus disease.44 Type 1 ROP is considered high-risk and is recommended for treatment, whereas type II is usually closely monitored.11Subsequent follow up and treatment are then determined based on ophthalmic findings and infant age.11
Complications of retinopathy of prematurity
There are many well-documented complications of ROP. They include tractional retinal detachment from fibrovascular proliferation, and subsequent vision and visual field loss, glaucoma, high myopia, retinal folds, tears and detachments, and macular dragging.45
Current standard of care and pitfalls
The current standard of care for Type 1 ROP is treatment of the avascular, hypoxic peripheral retina with laser, preferable to cryotherapy.43,44 This treatment is believed to treat hypoxic cells that upregulated VEGF and other angiogenic factors and/or indirectly to reduce the angiogenic stimulus to hypoxic retina and downregulate angiogenic signaling in order to reduce abnormal vessel growth, and later fibrovascular retinal detachment and blindness.44,46–48 The ET-ROP study showed that laser performed for high risk prethreshold disease (≥15% risk of progression to an unfavorable outcome from previously defined modeling49) resulted in a reduction of unfavorable visual acuity outcomes from 19.8% to 14.3% and unfavorable anatomic outcomes from 15.6% to 9.0% (Table 1).44,50,51 However, if treatment is initiated later, or is inefficient and/or incomplete, therapeutic results can be poor in some cases because of continued vasoproliferation and fibrovascular changes. Even with successful treatment and preserved retinal attachment, visual outcomes can be poorer than desired. The ET-ROP study reported that visual acuity was 20/40 or better in only 34.6% that underwent early treatment 6 years later.52–54 Possible causes of poor vision (≤20/200) despite a normal fundus include cortical blindness, other anterior and posterior visual pathway diseases that require further study, and neurovascular dysfunction at the retinal level.55–57
Unfortunately, while effective if performed appropriately, laser or, less frequently cryotherapy, requires proficiency in indirect ophthalmoscopy and scleral depression in neonates and is time intensive requiring at least 2 hours per laser treatment.58 Worldwide, there is currently a shortage of adequately trained ophthalmologists to diagnose and treat ROP.59 Teleretinal imaging is being used to diagnose and stage ROP in efforts to address shortage of ophthalmologists in certain areas.60 Additionally, some postnatal weight-gain algorithms have been developed and tested to identify infants at risk to develop ROP or severe ROP. However, these efforts will have no effect on the lack of available ophthalmologists for treatment,61–63 and studies are still evaluating its widespread use.64 However, it remains to be seen if efforts to address screening burden will also address the lack of qualified ophthalmologists to provide appropriate treatment.2,59 In some cases, despite early laser therapy or in those infants in whom late recognition of the disease occurred, ROP can progress to retinal detachments and other complications. Thus, early recognition and treatment is paramount to avoid late complications and maintain vision. Scleral buckles and vitrectomies are used to treat retinal detachment.45 Other treatment modalities are being tested to combat ROP and the longterm consequences.
Anti-vascular endothelial growth factor
The development of anti-VEGF agents has led to their widespread use in treating diabetic macular edema, macular edema from vein occlusions, proliferative diabetic retinopathy, neovascular glaucoma, neovascular age-related macular degeneration, and choroidal neovascular membranes.65,66 There are currently four anti-VEGF agents on the market delivered as intravitreal injections: pegaptanib, ranibizumab, aflibercept, and bevacizumab. While bevacizumab is not currently approved for intravitreal use, its off label use has been well tolerated and quite effective in treating the entities described above for over 10 years.67–69 Pegaptanib is a selective inhibitor of the VEGF165 isoform, while ranibizumab, a monoclonal antibody fragment, aflibercept, an engineered fusion protein fused to the Fc portion of human immunoglobulin G (IgG)1,
and bevacizumab, a monoclonal antibody, are all non-selective VEGF inhibitors.67,70–72 Ranibizumab is a slightly smaller molecule compared to aflibercept and bevacizumab, while aflibercept has the broadest spectrum of activity.73 Due to their ease of administration and mechanism of action, some of these agents have been proposed as potential therapeutic agents that could avert an emerging crisis due to a shortage of trained ophthalmologists available to treat ROP worldwide. In addition, many of the preclinical studies done for diabetic eye disease and age-related macular degeneration used models of OIR to test the effect of anti-VEGF treatments. Although OIR models do not completely translate to human ROP, some similarities in stresses and outcomes led to a movement from OIR to ROP. Using the representative rat model, it was found that inhibition of VEGFR2 signaling reduced neovascularization but did not inhibit physiologic retinal vascularization in an incompletely understood process. This suggested that inhibiting, but not completely abolishing VEGF signaling, would not only be safe for retinal vascular development but would also reduce vasoproliferation in ROP.26–28,74 This hypothesis was corroborated in part by an early, promising, interventional cases series and another randomized trial that have since paved the way for larger interventional studies.75,76
Several studies evaluating the efficacy of anti-VEGF agents in ROP have shown promise but lack longitudinal, extended follow up (Table 2). Furthermore, most of the studies have been relatively small. One such study that included 13 infants found that there was no difference in visual acuity and refractive error in those preterm infants treated with intravitreal bevacizumab or laser for type 1 ROP in zone I or II.77 This was also reported
in a study of 26 patients with stage 3 ROP treated with bevacizumab in whom involution of neovascularization, flattening of demarcation lines, and growth of differentiated vessels into capillary-devoid areas was noted.78 In a larger study of 152 eyes with stage 3+ ROP in zone I or II, intravitreal pegaptanib combined with diode laser photocoagulation outperformed laser therapy and cryotherapy in reducing rates of recurrence of stage 3 disease. This combination therapy also led to faster resolution of neovascularization following initial treatment.79 Additionally, a more recent retrospective study showed that bevacizumab with or without zone I sparing laser reduced unfavorable outcomes (i.e., retinal folds, disc dragging, retinal detachments, early refractive errors, and retrolental tissue obscuring the view of the posterior pole) from 22.7% to 0% as compared to laser alone.80 The Bevacizumab Eliminates the Angiogenic Threat of (BEAT)-ROP was a randomized, multicenter trial that evaluated the efficacy of bevacizumab in neonates with stage 3+ in zone I or II and found similar results for zone I to those seen with pegaptanib.76 However, bevacizumab did not show a significant treatment effect for zone II disease in this study.76 Additionally, a randomized, controlled clinical trial has shown that those infants treated with bevacizumab had statistically significant lower rates of very high myopia and spherical equivalent refractions compared to those treated with laser.81 Specifically, infants with zone I disease treated with bevacizumab had mean spherical equivalent refractions of -1.51 diopters (D) versus those treated with laser who had -8.44 D. This significant trend continued into posterior zone II (-0.58 D versus -5.83 D). Lower rates of very high myopia (≥-8.00 D) in infants with ROP in zone I and II was also reported following bevacizumab injection versus laser treatment (3.8% versus 51.4%, 1.7% versus 36.4%, respectively).81 Other retrospective reports have shown similar results.82–84 Furthermore, a recent Cochrane meta-analysis that reported reduced myopia and retinal detachment with anti-VEGF agents, emphasized that the quality of evidence did not allow authors to fully rule out bias. The Cochrane review concluded that due to insufficient data and an unbeknownst systemic safety profile that strong conclusions could not be made favoring routine use of anti-VEGF agents.85 While the BEAT-ROP trial has shown promising results of anti-VEGF therapy when compared to peripheral laser photocoagulation for stage 3+ disease, there are still many significant questions that must be answered before widespread use is adapted into clinical practice.76 The current recommendations of the American Academy of Pediatrics include considering bevacizumab for zone I, stage 3 with plus ROP and treatment of type 1 ROP with laser.11
The role of anti-VEGF agents in treatment of ROP has insufficient evidence regarding the agent, dose, treatment regiment, efficacy versus laser and safety, to yet recommend widespread acceptance to preterm neonates who are medically fragile. While there have been no long-term safety studies in premature infants who are still undergoing development, there have been several studies addressing safety in adults undergoing repeated administration of anti-VEGF. A recent meta-analysis evaluated long-term safety in adults with diabetic macular edema and included a total of 1328 patients from four separate studies. The authors concluded that those patients who received the highest exposure of ranibizumab, monthly injections for 2 years, as compared to sham or laser treatment had an increased risk of death, cerebrovascular events, and vascular death (odds ratio of 2.98, 2.33, and 2.51 respectively).86 This was in contrast to an older Cochrane review published in 2014 that found no difference in systemic adverse events in those treated with anti-VEGF agents on an as-needed basis versus those who had not.87 These disparate results, we postulate, could be due to more stringent inclusion criteria of the first study requiring imposed monthly injections for two years, while the latter included studies with far fewer treatments. Thus, there are safety concerns in premature infants but to what effect remains unseen. Even though neonates are not usually more than one dose, there is evidence that current anti-VEGF doses administered to infants causes a greater effect than in adult, and may have adverse effects on organ systems and neural cognition in developing preterm infants.88
In premature infants, there is very little information on the safety profile of anti-VEGF therapy. Experimental evidence supports that VEGF is an important survival factor for glial and neural cells.89,90 In neonatal mice, anti-mouse VEGF 164 antibody has been shown to reduce brown fat.91 Thus, experimental evidence would suggest both local and systemic effects from treatment with potential long-term consequences. In infants, intravitreal bevacizumab reduces systemic VEGF concentrations for up to 2 months due to systemic leakage from the vitreous.91–93 In a small study including 11 infants followed weekly for a month, ranibizumab only suppressed plasma VEGF for up to 1 week but unfortunately had much higher rates of ROP recurrence than bevacizumab.94–96 This systemic absorption and pronounced VEGF neutralization raises concerns about effects on developing neural and vascular beds in the premature infant. The Canadian Neonatal Network study found that infants treated with bevacizumab had higher odds of severe neurodevelopmental disabilities compared to those infants treated with laser.97 The study was retrospective, relatively small in size and was not randomized, in that infants treated with bevacizumab had more medical issues and potentially prone to neurocognitive delays. Nonetheless, the study raises important safety concerns of anti-VEGF therapy.
There are well-documented immediate complications to anti-VEGF therapy in some neonates. Reports of anti-VEGF complications include peripheral retinal abnormalities (i.e., large, avascular areas, abnormal branching vessels, and/or shunt vessels) or posterior pole anomalies (i.e., hyperfluorescent lesions and absence of foveal avascular zones) compared to those treated with laser, as seen by fluorescein angiography.98 While this study was limited by short follow up time of 9 months and only 13 infants being enrolled in the study, the findings raise concerns for lasting negative architectural effects of anti-VEGF treatment.98 Additionally, infants treated with bevacizumab or ranibizumab have been reported to have recurrence of ROP between 4 and 35 weeks after initial treatment.95,96,99 Another small retrospective study that included 58 eyes noted that those infants requiring earlier bevacizumab treatment or had lower initial birth weights required significantly higher rates of rescue treatment.100 In the rat 50/10 OIR model, it was shown that inhibition of VEGF bioactivity using a neutralizing antibody led to later intravitreal neovascularization in part associated with activation of other angiogenic pathways.101 The late reactivation in infants also makes extended follow up a necessity compared to laser therapy in a world where providers are already difficult to find. Additionally, the risks of complications directly associated with the injection, such as endophthalmitis, are rare but real.102
Recombinant IGF-1 supplementation
As previously mentioned, the interplay between VEGF and IGF-1 may be a driving force in the development of ROP; lower serum levels of IGF-1 are seen in preterm babies that develop ROP.35,103 Moreover, serum IGF- 1 levels are known to precipitously drop following preterm delivery due to the loss of interaction between the fetus and mother in utero.104 Thus, groups have proposed supplementing preterm infants with IGF- 1 to in utero levels of infants of similar developmental ages (≤ 33 μ/L at 33 weeks postmenstrual age raises risk for any preterm morbidity 2.2 fold35) to promote physiologic retinal vascularization and reduce hypoxia resulting from large areas of avascular retina, which is a risk for the development of ROP.35 A recent phase II clinical trial evaluated continuous infusion of IGF-1 required to maintain serum IGF-1 levels (clinical trial NCT01096784) and did not reach its primary outcome measure of reduced severe ROP, but had important reductions in secondary outcomes such as bronchopulmonary dysplasia and severe intraventricular hemorrhage. A 2-year interim study is planned through 2018 (Section D, NCT01096784; PEDAL, NCT02386839). Several smaller studies had already shown safety of the proposed treatment.105,106 Furthermore, IGF-1 knockout mice have impaired retinal vessel growth and treating mice with OIR, which simulates ROP, with recombinant IGF-1 results in a decrease in retinopathy and faster overall developmental maturation.30,107 Unfortunately, this recent randomized trial with recombinant human IGF-1 did not meet its endpoint of reducing rates of ROP; however, this drug may become important in reducing other prematurity-related health problems.
Additional benefit from anti-oxidants such as D-Penicillamine, long chain fatty acids, or vitamin E in preventing ROP
Recent experimental evidence has shown a role of free radicals inducing retinopathy through NADPH oxidase-dependent reactive oxygen species generation and has been speculated to be a contributor to the initial development of angiogenesis and ROP.108–113 Several small clinical studies have shown some efficacy of reducing the frequency of ROP by neutralizing oxidative compounds. Once such agent, D-Penicillamine, is a potent anti-oxidant that has been shown in a small, non-randomized study to reduce ROP development in preterm infants who enterally received a 14-day course of the medication. Of note, there was no associated systemic toxicity associated with the treatment.7,114 While the mechanism is unclear, D-Penicillamine is thought to chelate pro-oxidant heavy metals thereby reducing free radical activity and subsequent retinal injury required to initiate ROP.7,115 Unfortunately, a more recent Cochrane meta-analysis found no benefit to D-Penicillamine administration to prevent ROP on currently available evidence.116 This will likely end any future role of the drug in ROP prevention.
Omega-3 and -6 polyunsaturated fatty acids are long chain fatty acids that must be consumed in diets and are transferred from mother to fetus during the third trimester. They are the predominant fatty acid in brain gray matter and retinal membranes.104 These fatty acids have been shown to prevent photoreceptor apoptosis in vitro in response to oxidative stress and are known potent anti-oxidants.117–119 In mouse pups with induced oxidative retinopathy whose mothers were fed with long chain fatty acids, these mice were shown to have revascularization of avascular retina and reduced neovascularization due to lower concentrations of inflammatory mediators. This resulted in attenuated endothelial cell activation.120,121 In a small, observation study from Poland, preterm neonates requiring total parenteral nutrition were given either a diet with fish-emulsion (contains docosahexaenoic acid) additive versus control.122 In the group receiving the fish extract, there was less need for laser therapy and more infants had increased rates of spontaneous regression of ROP.122 Regrettably, this did not result in better visual outcomes (i.e., improved visual acuity and visual evoked potentials).122 These results were subsequently confirmed with a small, randomized control trial from the same group.123 Thus, it is likely that supplementation with long chain fatty acids will have some role in reducing the risk of developing ROP in preterm, very low birth weight infants but its exact role is still unclear at this time.
Vitamin E is a free radical scavenger, and due to the aforementioned role of antioxidants in reducing ROP risk, has been tested in several large studies. While high doses of vitamin E have shown to decrease ROP risk and blindness, there are risks including of sepsis.124 Therefore, current Cochrane meta-analyses compiling 26 randomized clinical trials recommended against high dose vitamin E supplementation due to lack of supportive evidence and risks associated with it.125 Therefore, vitamin E is not recommended for preventing ROP.
Conclusion
In conclusion, the role of anti-VEGF therapy in ROP treatment is controversial but from available evidence, it appears that regulation of the VEGF signaling pathways may have a place in facilitating physiologic angiogenesis and inhibiting pathologic angiogenesis. However, there are safety concerns that have not been fully evaluated in the small-scale studies done to date. In infants being evaluated for anti-VEGF therapy, informed consent is paramount as long-term effects are unknown. Currently, the American Academy of Pediatrics (AAP) and American Academy of Ophthalmology (AAO) recommends laser for type 1 ROP and consideration of the use of anti-VEGF agents in infants with zone I ROP, with stage 3 and plus disease.11 Currently clinical trials (NCT02390531 and NCT02375971) are testing the effect of several doses and of anti-VEGF versus laser. The evidence from these randomized controlled studies will hopefully provide greater insight into safe and effective treatments for ROP. However, future treatments may need to target pathways involved in pathologic vascular signaling without adversely affecting neural development







