Diabetic macular oedema (DMO) is a complication of diabetic retinopathy, a microvascular ocular complication of diabetes mellitus.1 The blood–retinal barrier (BRB) is a specialised structure, which tightly regulates, maintains and balances the levels of electrolytes, protein and fluid in the retinal tissues – a function that is central to the maintenance of normal visual acuity (VA).2–4 Disruption of the BRB’s function is believed to be instigated by complex pathological processes, including elevated levels of growth factors (vascular endothelial growth factor [VEGF]) and cytokines, advanced glycation end product formation, inflammation, hyperglycaemia, and pericyte loss. These alter the permeability characteristics of the retinal endothelial cells and compromise the integrity of the BRB.5 A dysfunctional BRB and the resulting increased vascular permeability allow abnormal inflow of fluid and molecules into the neurosensory retina, which exceeds outflow.6 The consequence is an accumulation of metabolites including proteins and lipids in the extracellular space, leading to the sequestration of fluid within the retinal layers which, in turn, results in retinal dysfunction and loss of central vision.6–8
Among the estimated 415 million people, globally, who suffer from diabetes mellitus, about 6.8% are afflicted with DMO.3 With a rising incidence of both type 1 and type 2 diabetes mellitus, and the increasing life expectancy of populations in the developed and developing world, the prevalence of DMO is predicted to rise even further in the coming years.9 It is expected that, without treatment, within 2 years of developing DMO, around 50% of patients may experience a decrease in VA by ≥2 lines in the affected eye.10
The management of DMO has evolved over the years, and the main available treatments are laser therapy, anti-VEGF therapy, ocular steroids and surgery.6,11–14 Anti-VEFG therapies revolutionised the treatment of DMO and have become the first-line treatment for patients with centre-involving DMO.15–17 Anti-VEGF agents, such as bevacizumab, ranibizumab and aflibercept, were developed on the basis that VEGF plays a vital role in promoting vascular permeability, which is an underlying factor in DMO.7,17 Anti-VEGF agents target and inhibit the effects of VEGF, thereby significantly improving functional outcomes.1,6,17 However, around half of all eyes treated with anti-VEGF therapies (55–67%) have a suboptimal response owing to incomplete resolution of retinal oedema despite multiple and frequent injections.3,16 Because the anti-VEGF treatments are monoclonal antibodies that target only VEGF, it has been suggested that the retinal oedema in eyes of partial or non-responders are a consequence of other inflammatory mediators that also play a pathogenetic role in the development of DMO.3,17
Corticosteroids have a variety of effects relevant to DMO management such as the suppression of inflammation and angiogenesis, and a reduction in excessive vascular permeability.3,12,18–21,22 However, the benefits that arise from the use of corticosteroids need to be weighed against well-known side effects of raised intraocular pressure (IOP) and progression of cataract.14,22
Current methods of delivering corticosteroid treatments include intravitreal injection and sustained-release drug delivery implants.22,23 Corticosteroids, such as triamcinolone acetonide administered by intravitreal injection, have been widely used to treat DMO and have been shown to enhance VA.22 However, this preparation has a short lifespan in the vitreous reservoir and requires frequent injections,23 which negatively affects the patient’s quality of life.5,15 Sustained-release drug delivery implants include Ozurdex® (Allergan Inc., Irvine, CA, US), an intravitreal dexamethasone implant that releases dexamethasone gradually for up to 6 months,13 and ILUVIEN® (Alimera Sciences Inc., Alpharetta, GA, USA), an intravitreal implant that was specifically developed to address the lack of long-term steroid dosing within the eye by providing sustained release of fluocinolone acetonide (FAc). This was demonstrated in the FAMOUS study where aqueous levels of FAc were measured after administration of ILUVIEN®; after an initial peak at month 1 (2.17 ng/ml) steady-state levels were sustained between 1.0–0.5 ng/ml from month 6 through to month 36.23 It offers the benefit of sustained delivery of submicrogram levels of intravitreal corticosteroids for periods of up to 3 years, thus radically reducing the number of injections required.15,23
In Europe, the FAc intravitreal implant is licensed for the treatment of vision impairment associated with chronic DMO insufficiently responsive to available therapies.24 The implant is also available in the US, where it is licensed for the treatment of DMO in patients who have been previously treated with a course of corticosteroids and did not have a clinically significant increase in IOP.25 Hence, the FAc intravitreal implant is licensed for the treatment of DMO that persists or recurs despite treatment.
This article reviews the clinical effectiveness and safety of the FAc intravitreal implant from real-world studies presented at the Association for Research in Vision and Ophthalmology (ARVO) 2018 Annual Meeting, as well as summarising its pharmacological and pharmacodynamic properties.
Review
Drug and device
The FAc intravitreal implant is a non-biodegradable micro-implant designed to release the corticosteroid FAc into the vitreous cavity over a prolonged period of time.23,24 The implant, which measures 3.5 mm in length and 0.37 mm in width, is made of a polyvinyl alcohol (PVA) matrix encased in a polyimide tube, which is coated with silicone adhesive at one end and permeable PVA at the other.24 It is inserted into the eye via the pars plana through a preloaded 25-gauge needle inserter. The implant contains 0.19 mg of FAc, which is released via its permeable PVA end at an average dose of 0.2 μg per day for up to 36 months.24,26
Pharmacokinetic and pharmacodynamic properties
FAc is a corticosteroid anti-inflammatory agent.27 Inflammation disrupts some biological pathways and enhances others. Corticosteroids are known to inhibit inflammation through reducing oedema formation, fibrin and collagen deposition, capillary dilation or proliferation, and leukocyte migration.12,23 They therefore inhibit prostaglandin and leukotriene synthesis and affect other pathways, including the intercellular adhesion molecule-1, interleukin-6, VEGF-A and stromal cell-derived factor-1 pathways.27 Steroids can act to restore tight junctions through increasing key junctional protein expression, reducing vascular permeability and cellular layer integrity.27
Clinical effectiveness
FAME trials
The efficacy of FAc in DMO was demonstrated in the FAME (Fluocinolone Acetonide for Diabetic Macular Edema) A and B trials, in which patients who had persistent DMO despite at least one macular laser treatment were randomly assigned to the FAc intravitreal implant 0.2 μg/day (licensed dose; n=375), 0.5 μg/day (n=393) or sham injection (n=185).28,29 In both trials, a significantly higher proportion of patients treated with the low- or high-dose FAc implant experienced improvement from baseline in best-corrected VA at months 24 and 36, when compared with those receiving the sham injection. Foveal thickness was also reduced in the FAc groups when compared with the sham injection group at all time points, except at 36 months.28,29
Real-world data sets
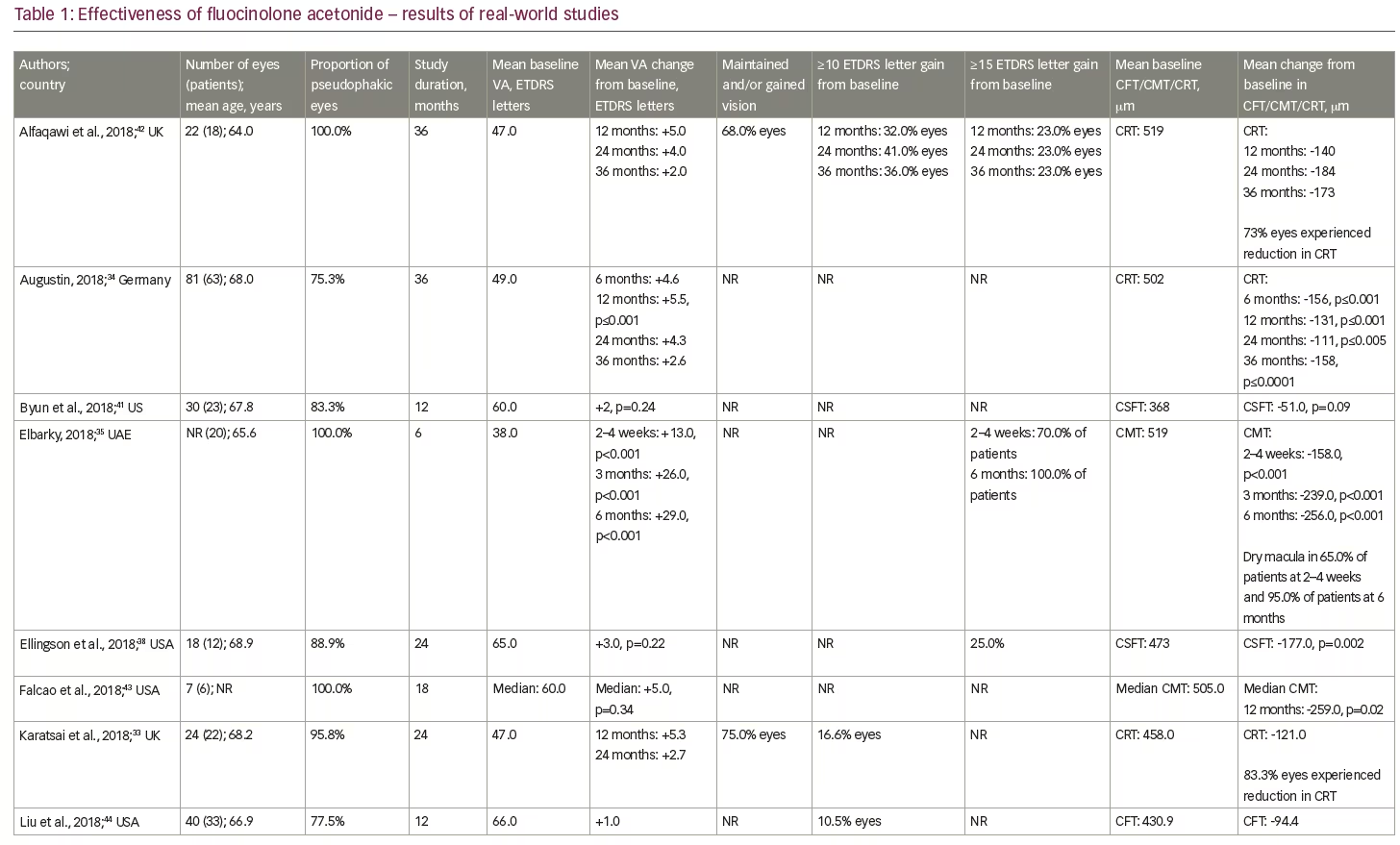
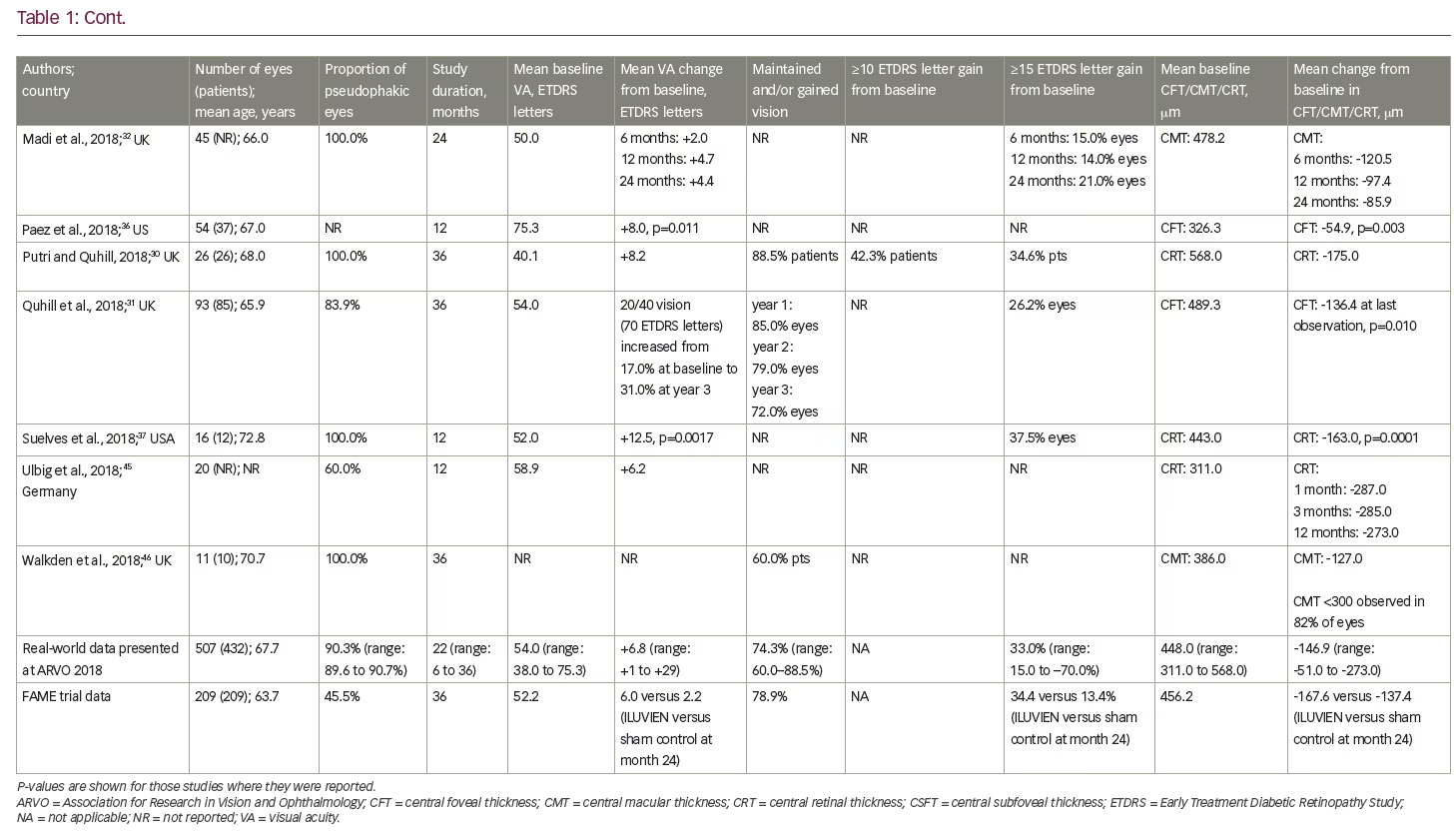
To date, real-world clinical effectiveness data on the use of FAc in persistent or recurrent DMO have generally been consistent with those at similar time points in the two FAME trials. Table 1 summarises the relevant real-world studies presented at the ARVO 2018 conference, with key findings from some studies discussed in more detail below. These were a series of small case studies from around the world, in which the findings were remarkably consistent, with most showing improvements in VA and reductions in central retinal thickness (CRT).30–46 Table 2 summarises the DMO studies presented at the conference demonstrating the effect of FAc on treatment burden.30,31,34,37–42,44,46
European real-world data sets
Putri and Quhill presented a retrospective review of electronic medical records from 26 patients (26 eyes) and showed that the FAc implant increased best-recorded VA by approximately 8.2 ETDRS letters (from a baseline of 40.1 letters) and decreased CRT by 175.0 μm (from a baseline of 568.0 μm).30 88.5% of patients maintained or gained VA and 35% gained ≥15 letters. The average number of DMO treatments post-FAc decreased: anti-VEGF injections decreased from 7.7–2.9 treatments and macular laser decreasing from 1.1–0.4 treatments.
In another 3-year retrospective audit conducted across 14 UK sites and involving 93 eyes from 85 patients, Quhill et al. also assessed VA outcomes.31 They showed the proportion of eyes achieving driving vision nearly doubled at year 3 (from 17% at baseline to 31% at year 3), 72.0% of eyes maintained or gained VA and 26.2% of eyes gained ≥15 letters. Reductions in mean central foveal thickness (CFT) were also reportedly maintained at year 3 with an overall reduction of 136.4 μm from 489.3 μm at baseline. There were also reductions in the average number of DMO treatments (from 5.4 pre-FAc to 4.1 post-FAc).
Augustin presented the 3-year results from a German retrospective study (Retro-IDEAL) involving 81 eyes (63 patients) and showed improvements in mean VA at month 6 (+ 4.6 letters) were maintained to year 2 (+ 4.3 letters) before declining slightly at year 3 (+ 2.6 letters).34 This was accompanied by a decrease in central macular thickness (CMT) of 158 μm from a baseline of 502 μm.
Middle East real-world data sets
Elbarky presented the first results from 20 patients treated in the United Arab Emirates.35 Six months after treatment with the FAc implant, mean VA improved by 29 letters from a baseline of 38 letters and mean CMT decreased by 256 μm from 519 μm at baseline. All patients gained ≥15 letters at month 6 and 95% of patients achieved a CMT of <350 μm.
US real-world data sets
Paez et al. assessed the effectiveness of FAc in a retrospective, observational case series in 54 eyes from 37 patients.36 After 1 year, VA was shown to improve by 8.0 letters, from a baseline of 75.3 letters, and mean CMT decreased by 54.9 μm from a baseline of 326.3 μm. Similar improvements were reported by Suelves et al. with a mean CRT improvement of 150 μm from a baseline of 443.0 μm and a mean VA improvement of 12.5 letters from a baseline of 52.0 letters.37
Longer-term results, up to 2 years, were presented by Ellingson et al.38 This was a retrospective review of 18 eyes (12 patients) with good baseline VA (65 ETDRS letters pre-FAc implant). The authors reported mean improvements in VA (+3 letters from a baseline) and central subfoveal thickness (-177 μm from a baseline of 473.0 μm).
Concerning treatment burden, analyses of two real-world studies (PALADIN and USER) by Gonzalez A39 and Singer40 consistently showed significant reduction in the frequency of DMO treatments post-FAc implant (USER: from one treatment every 2.9 months pre-FAc implant versus 14.3 months post-FAc implant; and PALADIN: one treatment every 3.7 months pre-FAc implant versus 7.9 months post-FAc implant). More than 50% of eyes in both studies did not require additional DMO treatments post-FAc. These findings were complemented by a retrospective chart review by Byun et al. in 30 eyes (23 patients).41 The authors showed the frequency of treatment decreased from one injection every 2.6 months pre-FAc implant to one injection every 8.8 months post-FAc implant. The FAc implant led to a reduction in the mean number of ophthalmology-related office visits, which decreased from 12.3 visits pre-FAc implant to 9.3 post-FAc implant.
Safety
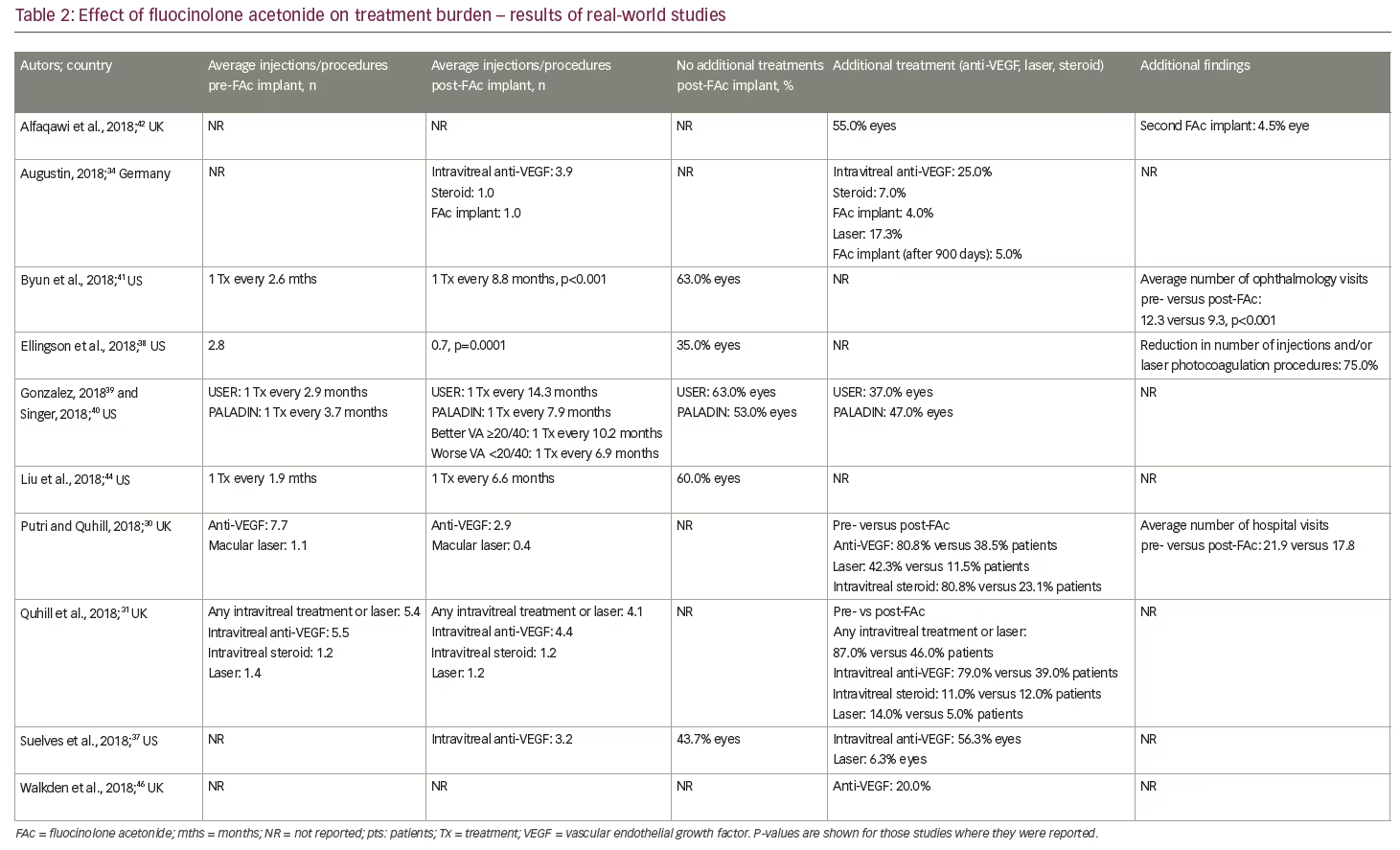
As with all intravitreal steroids, one of the main safety concerns is raised IOP. Global cumulative data for the FAc implant, obtained from Periodic Safety Update Reports, documents a total of 12,407 patient-eyes had been treated up to August 2017. Based on spontaneous safety reports to authorities, and through searches of literature, non-interventional studies and other sources, the incisional IOP-reducing surgery rate was less than 1% (n=41/12,407; 0.33%).47 Some caution is needed, however, when interpreting these results, as spontaneous reports are voluntary and, therefore, reporting rates are expected to be lower than actual patient events in real-world practice. Indeed, in the European post-authorisation safety study (the ILUVIEN Registry Safety Study; IRISS) conducted in the UK, Germany and Portugal, which included 593 eyes in 563 patients, emergent IOP medication was required in 23.3% of eyes and incisional surgery in 2% of eyes during a mean follow up of 471.2 days.48 This demonstrates that elevations in IOP were effectively managed with IOP-lowering drops in the majority of cases.
Current real-world evidence has revealed no additional safety concerns for the FAc implant in patients with persistent or recurrent DMO (Table 3). Unlike the FAME trials, in which a previous history of an IOP rise secondary to steroid use was an exclusion criterion, these real-world studies included patients previously exposed to steroids and with a history of IOP events. Indeed, the US label specifies the use of the FAc implant for 'the treatment of DMO in patients who have been previously treated with a course of corticosteroids and did not have a clinically significant rise in intraocular pressure'.25 Real-world data from the US show that IOP levels pre- and post-administration of the FAc implant were not statistically different;31,34,36,38,44 these data demonstrate the value of prior corticosteroid exposure in mitigating the risk of uncontrolled IOP rises. The predictive value of prior IOP events has also been studied and those patients who did not have IOP-related events prior to the FAc implant were shown to have a lower likelihood of subsequent IOP complications.49 This type of patient is, therefore, less likely to experience a clinically significant rise in IOP following FAc treatment,49 which provides some reassurance to the treating physician. It is also notable that a number of cohort studies conducted in the US36,38,44,50 reported only marginal increases in IOP and a minority of eyes (8.3–17.5%) required topical medication to control IOP rises. In one case series, a single patient required surgical intervention.50 In the retrospective study by Augustin,34 mean IOP was stable between baseline (15.8 mmHg) and year 3 (15.6 mmHg). IOP-lowering drops were required in 49.4% of eyes during the study, with 22.2% requiring IOP-drops prior to injection of the FAc implant. In the majority of cases, elevated IOP was effectively managed with IOP-lowering drops alone.34 Similar findings were reported by Quhill et al.31
Another important safety consideration when using the FAc implant, like any corticosteroid, is cataract formation. The FAc implant is licensed for use in patients where DMO persists or recurs despite treatment and is indicated for use in patients with no restriction on lens status.24,25 This is highlighted in Table 1, which shows that the FAc implant is used predominantly in pseudophakic eyes (90% of cases), although there is some use in phakic eyes (~10% of cases; range, up to a maximum of 40% in some centers45). In the UK, treatment is restricted to pseudophakic eyes only, although this is based on cost effectiveness (that is, value for money to the National Health Service) rather than clinical judgment.51
Expert opinion and conclusions
Although the introduction of anti-VEGF therapy has been highly effective in reducing the visual morbidity associated with DMO, there is a continuing unmet need as suboptimal treatment response remains an important cause of sight impairment due to this condition.1,52,53 Even among those patients who do respond to anti-VEGF therapy, the burden of care is high owing to the need for repeated injections over many years, which in turn, increases the risk of complications such as endophthalmitis.5 A single FAc implant can last for up to 3 years and its sustained action can also help to reduce the burden of visits and treatments; furthermore, it offers an alternative when DMO has been insufficiently responsive to anti-VEGF agents.
Consistent with the findings from the pivotal FAME trials and additional randomised controlled trials,28,29,54 the evidence from real-world studies supports the view that the FAc implant offers an effective treatment alternative in patients with persistent or recurrent DMO. Indeed, in the FAME studies the primary endpoint was an improvement in VA of ≥15 letters from baseline. In Table 1 the average duration of follow up was 22 months and 33.0% of patient eyes gained ≥15 letters at this time point. At a similar time point in FAME (at 24 months), 34.4% of patients gained ≥15 letters. Similar comparisons were performed for VA and CFT, and these revealed similar findings – CFT decreased by 146.9 μm and 167.6 μm (real-world and FAME, respectively); and VA improved by 6.8 letters and 6.0 letters (real-world and FAME, respectively).
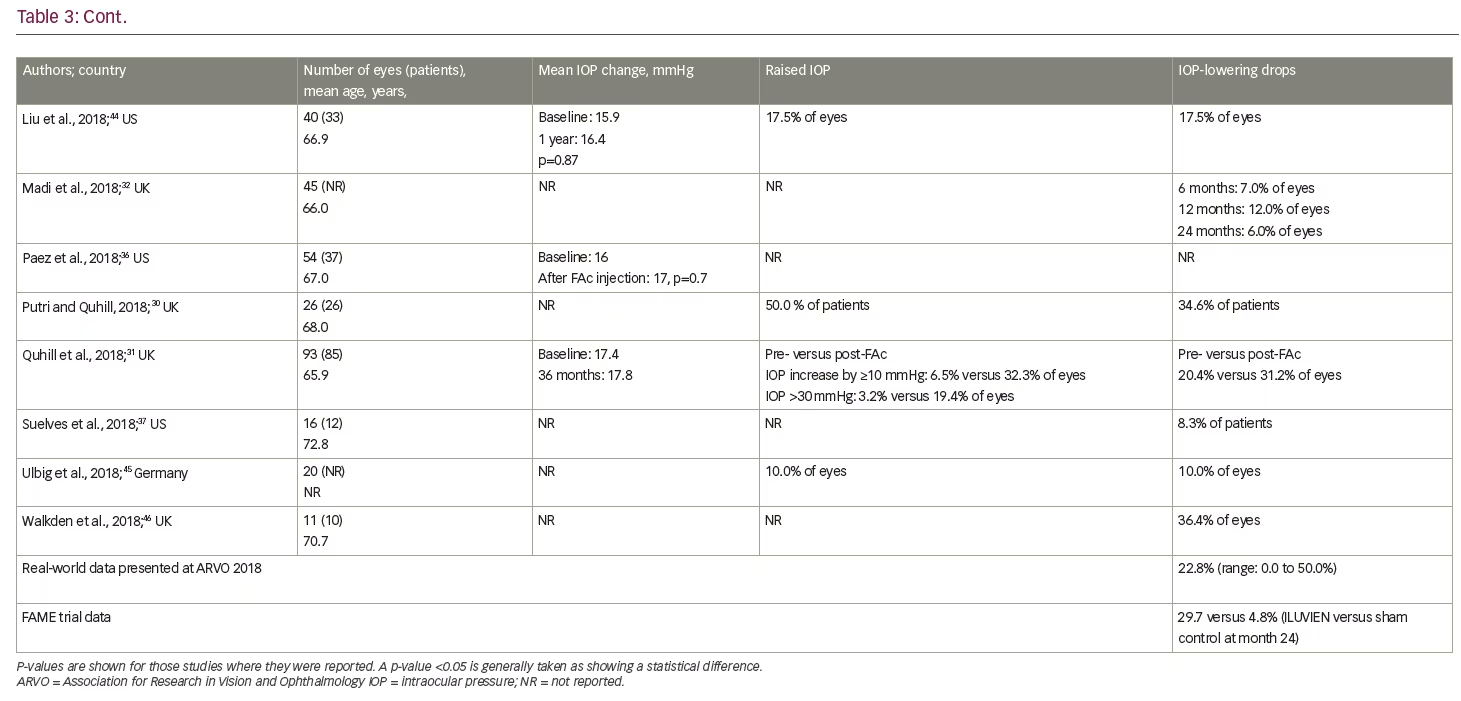
The data presented at ARVO 2018 also provided some new insights on the effectiveness of the FAc implant in the efficient running of a clinic. The studies by Byun et al.,41 Singer,40 Gonzalez39 and Liu et al.44 assessed treatment burden and showed the frequency of DMO therapies decreased after the FAc implant, with one treatment required every 2.8 months (range: 1.9–3.7 months) before and 9.4 months (range: 6.6–14.3 months) after the implant was administered. In terms of safety, the studies from the US were able to confirm that IOP events occurring pre- and post-administration of the FAc implant were not statistically different31,34,36,38,44 and where an IOP rise occurred, they were only marginal increases in a minority of eyes (8.3–17.5%).36,38,44,50 Furthermore, across all cases studies, it was clear that when an increase in IOP occurred, it was manageable in the vast majority of cases with IOP-lowering drops alone.34
The advantages of a reduced treatment burden with a low risk of increased IOP are important attributes. The consistent reporting of improvements in VA and retinal anatomy by numerous centres at the ARVO 2018 Annual Meeting32,36,38,43,45,46 is testimony to the value of this therapy in the treatment of persistent or recurrent DMO.30,31,34,35,37,42 In addition, emerging evidence shows that patients with DMO, who were insufficiently responsive to intravitreal anti-VEGF drugs, dexamethasone implants and/or focal laser therapy, experienced additional improvements in function after administration of the FAc implant,45 which raises the important question whether current use of the FAc implant in clinical practice (that is, once DMO has been defined as persisting or recurring after treatment) is optimal.
Cataract progression and elevated IOP are well-known side effects of corticosteroid therapy and they need to be considered by the treating physician when choosing to use the FAc implant. The physician should also consider the practicality of the sustained-release implant compared with repeated intravitreal injections as it may be helpful in improving the efficiency of very busy DMO clinics.55 The FAc data presented at the ARVO 2018 Annual Meeting demonstrated similar safety and tolerability outcomes; specifically, the percentage of patients requiring medications to control rises in IOP were similar to those reported in the FAME studies at a similar time point and this is important because the implant was designed to last 3 years. The majority of patients (62%) did not require any IOP-lowering medication during the 3-year FAME studies,28,29 and those who did were nearly all managed with IOP-lowering drops. This has now been confirmed in real-world practice. Nonetheless, as there is a risk of an IOP rise, it is important that patients receiving the FAc implant are monitored quarterly, so that IOP is checked regularly.
DMO is a life-long disease requiring regular review and interventions. With anti-VEGF therapies, the current treatment paradigm is one of frequent review visits and intravitreal injections and this imposes a huge cost and treatment burden on healthcare systems. An evaluation of healthcare use among patients in the UK receiving the FAc implant56 suggested that it is a cost-effective treatment and these findings are in agreement with other cost-effective reports in the literature.57–60
Limitations
The current report compares the outcomes achieved from a collection of case studies presented at ARVO 2018 and represents data that is still being collected. Some of the cases have a relatively short follow up (up to 6 months) and this limits the comparisons made with outcomes in the FAME studies. Also, the number of centres with patients reaching a 3-year follow up is starting to increase and this will allow meaningful indirect comparisons as these data sets start to mature. A further limitation that influences the interpretation of findings is the difference in treatment indication in the US and Europe. In Europe, the FAc implant is generally used after a prior course of anti-VEGFs, whereas in the US its use follows a prior short-acting corticosteroid. This difference would explain some of the better baseline VA and CFT values, which will influence the magnitude of reported outcomes. These differences are also quite different from the FAME studies where laser was the last DMO therapy prior to treatment with the FAc implant. Lastly, the patients being treated in real-world practices are quite different to those in the FAME randomised controlled trials and this is reflected by the prior DMO therapies used and also the patient demographics (i.e., a higher mean age, a wider range of starting VA values and the high proportion of patients with a pseudophakic lens at baseline).
Summary
The growing body of data strongly supports the effectiveness and safety of the FAc implant as a long-term treatment option for persistent and recurrent DMO. With ongoing collection of larger sets of real-world data, evidence of the benefits and risks of the FAc implant continues to accrue and should further demonstrate its role in the long-term management strategy of DMO.







