Diabetes mellitus (DM) is a growing socio-medical problem because of its increasing prevalence and diabetic complication rate. This is also the reason for the large amount of money spent on diabetes treatment and on diabetic complications despite continually improving diabetes care.1,2 The International Diabetes Federation’s latest study has shown the number of people with diabetes rise from 382 million worldwide in 2013 to 592 million in 2035.3
One of most common microvascular diabetic complications is diabetic retinopathy (DR). It is a most common reason for blindness in developed countries. Sight-threatening DR can be avoided when identified early and cured in a timely fashion.4–7 This is the reason for diabetic screening implementation.
Rules of screening in medicine were established by Wilson and Jungner in 1968 and accepted by the World Health Organization (WHO).8 The basic principles for disease screening were as following:
- The condition sought should be an important health problem.
- There should be an accepted treatment for patients with
recognised disease. - Facilities for diagnosis and treatment should be available.
- There should be a recognisable latent or early symptomatic stage.
- There should be a suitable test or examination.
- The test should be acceptable to the population.
- The natural history of the condition, including development from
latent to declared disease, should be adequately understood. - There should be an agreed policy on whom to treat as patients.
- The cost of case finding (including diagnosis and treatment of
patients diagnosed) should be economically balanced in relation
to possible expenditure on medical care as a whole. - Case finding should be a continuing process and not a ‘once and
for all’ project.
These rules could be adapted for DR screening. The first attempts were made in 1989, when the St Vincent Declaration was made. According to this declaration, reduction of blindness due to DR by one-third in the following 5 years was planned.9 The next attempts on DR blindness reduction were established in 2005 in the Liverpool Declaration.10 The consensus was to reduce blindness due to DR by 2010, by DR systematic screening covering at least 80 % of the people with diabetes population with the use of trained personnel and access to the proper therapy.
Aim of Study
The aim of this study is to review current worldwide DR screening programmes and studies performed by technicians or primary care physicians (not ophthalmologists), quickly and simply.
Methods
This is a review study. A PubMed platform search was performed, using the following keywords: diabetic retinopathy screening programme, telemedicine and DR, prevented diabetic vision lost to find clinical trials or review studies of current DR screening programmes. We found 57 articles of which finally we analysed 45. English language was preferred, at least for abstracts.
Results
UK
The UK comprises England, Wales, Scotland and Northern Ireland. Each of them started to screen DR and developed their own national DR screening programmes. These services are based on digital colour eye fundus photography and vary in details.11–15
England
Wales
The Diabetic Retinopathy Screening Service for Wales (DRSSW) was started in 2002 to detect any kind of DR, especially sight-threatening DR.17,18 The DRSSW is a mobile screening service with the following protocol of screening: visual acuity, two-field 45° digital photographs (one in macula centre; one nasal) after 1 % tropicamid mydriasis and graded by retinal professionals. Patients with diabetes aged 12 or more and not involved in other screening programmes are covered by this service. Patients with sight-threatening DR are referred to a hospital retinal service. Patients with mild states of DR are advised to improve metabolic control of diabetes. This programme consists of 30 photographic teams and 220 locations in Wales and uses Canon DGi digital cameras. Photographs are graded according to the DRSSW protocol based on the National Screening Committee19 and the European screening handbook.20 Rate of patient cover is 80 %.14
Scotland
The Scottish National Diabetic Retinopathy Screening (DRS) service launched in 2006.21 Patients with diabetes 12 years and over are automatically identified according to the database of the Scottish Care Information-Diabetes Collaboration and due to this system patient coverage is above 99 %. Screening examination is based on a single, central 45° photograph with mydriasis if required. In case of complications with photographs, slit-lamp is performed. The grading system is centralised.22 Tests with good results with software for automated quality assessment and microaneurysm/dot haemorrhage have been carried out.13,23
Northern Ireland
The Northern Ireland Diabetic Retinopathy Screening Programme (NIDRSP) started in 2002. Its methodology is similar to the DRSSW. It is based on digital eye fundus colour photography with selective pupil mydriasis in patients under 50 years of age. This programme covers all patients with diabetes 12 years of age or over, with patient identification sent to the programme by the GP. Trained readers grade photographs.15
US
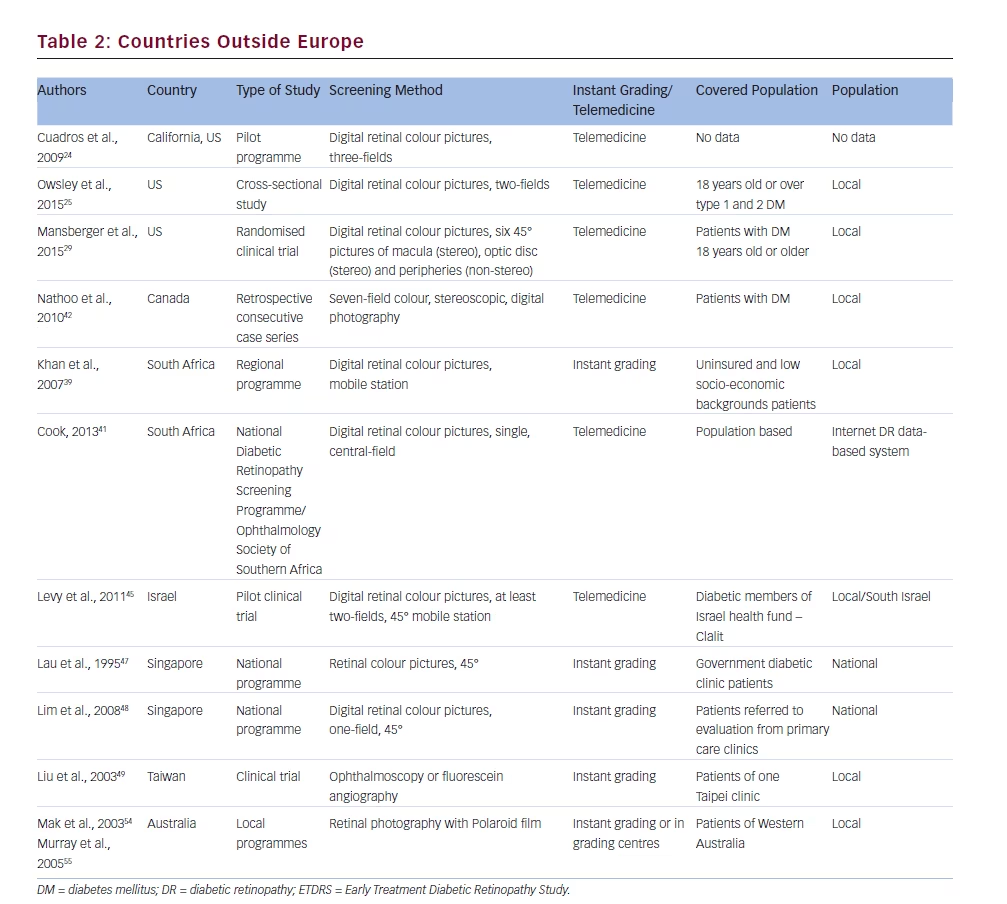
Cuadros et al. presented a study on the EyePACS (picture archive and communication system) programme developed in California.24 They used a licence-free system of taking pictures of the retina and sending them to a grading centre to assess retinopathy status. Pictures were taken by any kind of fundus cameras, but the non-mydriatic type was preferred, for instance, Canon CR-DGi or Canon CR-1. Certified EyePACS photographers took one picture of the external surface of the eye and three pictures of the retina, as follows: optic disc and macula, picture centred on optic disc and picture of the macula and temporal part of the retina. Retinopathy grading system reviewers performed grading of retinopathy. This programme was in operation in California in 2005 to 2006 and expanded to elsewhere in 2008.
We also found research on telemedicine reading of digital photographs of eye fundus of patients with diabetes.25 Patients 18 years of age or over with DM type 1 or 2 were screened. Trained photographers took three photos of the fundus of each eye: one anterior segment and two of retina located nasally and temporally and subsequently read by trained readers using telemedicine. They graded photos according to the British National Health Service’s DR grading classification system.26
The American Diabetes Association suggests that seven-field stereo 30° is superior to non-mydriatic digital photography and the second method should only be used for situations where mydriasis does not allow it.27
Analysis of the cost-effectiveness of screening DR in low socio-economic status areas has also been performed.28 This research was carried out for 17 months with the use of digital photography of the retina and grading by a telemedicine centre. There are no data on the exact condition of patients’ eye examinations.
Mansberger et al.29 performed a study on checking the usefulness of long-term telemedicine DR screening compared with traditional eye examination. They screened patients with DM 18 years of age or older. One study group was examined traditionally and the second with the use of a non-mydriatic camera and six 45° photographies: stereo on macula and optic disc; non-stereo on peripheries. The researchers concluded that telemedicine increased the percentage of eye examinations and could be a useful method for DR screening.
France
The first telemedical screening for DR started in 2002 in the Paris region and was conducted by Massin et al.30,31 They used a non-mydriatic fundus camera by Topcon and performed five-colour photographs of 45° of the central (macula and optic disc) and peripheral retina without pupil dilatation taken by orthoptists. The photographs were compressed and sent via the Internet to the grading centre, where quality of pictures and DR status was assessed. They used Topcon software for that process. The study was active for 18 months.
The Ophthalmology Diabetes Telemedicine Network (Ophdiat), a programme for DR screening, (created 2004; covering the Paris region).32 They used non-mydriatic fundus cameras (Canon or Topcon) and took 45° two-fields colour photos of the retina (one centred in the macula; the second centred in the optic disc). The pictures were taking by orthoptists or nurses and compressed to JPEG format and sent via the Internet to the grading centre, where DR status was assessed. Patients with moderate or more advanced stages of DR, or with unclear pictures, were referred to an ophthalmologist. All other people were screened once a year.
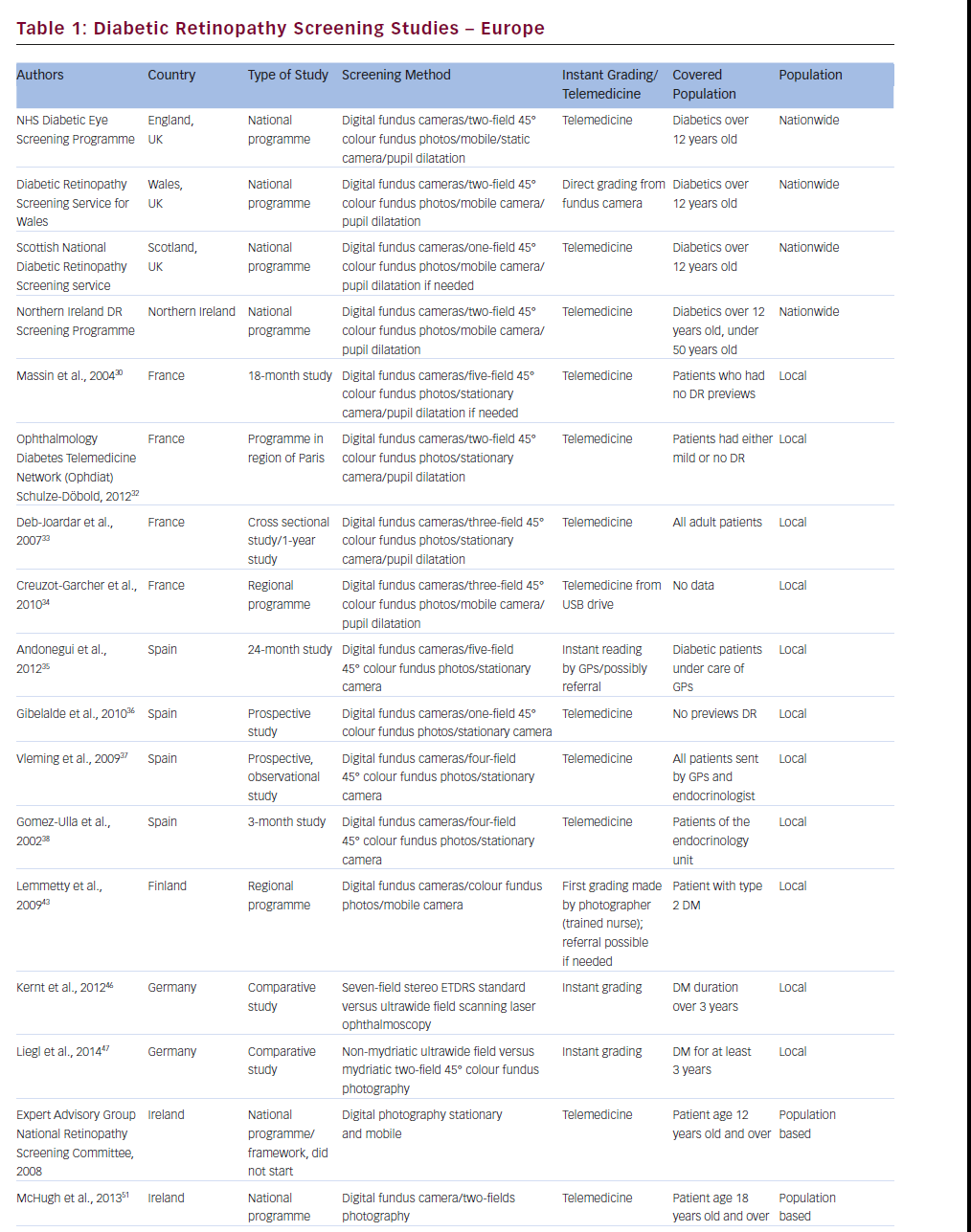
In 2003, Deb-Joardar et al. started university hospital screening for DR with the use of digital fundus camera. According to the protocol of this trial, three-field colour photographs were taken by ophthalmology residents after pupil dilatation with 1 % tropicamide and graded by a retina-specialised ophthalmologist.33
Creuzot-Garcher et al. conducted mobile screening for DR from 2004 to 2007 in three low medicalised areas of France, as defined by the Regional Health Agency (Observatoire régional de la santé) criteria. They used a truck fitted with a non-mydriatic fundus camera and an orthoptist took three photos of the retina: one centred in the macula, one nasal to the optic disc and one temporal to the macula. Pictures were stored using a USB flash drive and graded by an ophthalmologist at Dijon University Hospital. This campaign was designed to improve the coverage of diabetic patient screening to detect early a sightthreatening retinopathy.34
Spain
Andonegui et al. presented a 24-month Spanish study on DR screening based on digital retinal images performed and initially graded by GPs.35 Five pictures of each fundus with non-mydriatic Topcon fundus camera were obtained, taken by trained GP nurses and graded by trained GPs. When the interpretation of pictures was uncertain, GPs sent the patient to the ophthalmologist to assess retinal status. The authors suggest that proper GPs training can improve DR screening and save patients’ sight.
Gibelalde et al. performed a study on DR screening based on retinal digital photography with the use of a non-mydriatic Topcon fundus camera.36 They took single 45° central retinal pictures and sent them to the retinal department to assess DR status. They concluded that nonmydriatic retinography is a useful tool for DR screening as well as for other eye diseases.
Vleming et al. performed a study on DR screening based on four nonmydriatic eye pictures.37 They used a non-mydriatic Topcon fundus camera and the optometrists took three pictures of retina (one of the centre, one of the superior and one of the nasal periphery) and one picture of the front part of the eye. Photographs were sent to the retinal department, where they were assessed. They suggest that digital photography with telemedicine can be effective in DR screening.
Gomez-Ulla et al. performed a short study on DR screening with the use of a non-mydriatic fundus camera.38 Technicians took four digital images of the retina: one of the macula, one of the optic disc and one superior and one inferior retina. Pictures were sent via Internet to the central server and then were assessed by ophthalmologist. They suggest that digital photography and telemedicine is useful for DR screening.
South Africa
The first DR screening study using digital retinal photography in South Africa was created by Khan et al.39 They used a mobile fundus camera by Canon. The photographs were taken by a trained technician and graded by a trained doctor with ‘ophthalmic experience’. They extended their study till 2010.40 They concluded that digital imaging of the retina with non-mydriatic cameras is effective and cost-effective for DR screening in Africa.
The Ophthalmology Society of South Africa developed a DR-screening programme based on single, central colour photography of the retina.41 They adapted the Scotland DRS grading system, which is adequate for direct ophthalmoscopy as well for digital fundus imagining. They created a closed Internet-based DR database and patient-held examination record. Their programme has quality assurance conducted in cooperation with the Scotland DRS screening programme.
Canada
We found a study on DR screening in a rural region of Canada via telemedicine performed by Nathoo et al.42 They conducted a retrospective study on DR screening based on the Early Treatment Diabetic Retinopathy Study (ETDRS). Colour, retinal, stereoscopic, seven-field digital photographs were made using a Topcon fundus camera. Retinal pictures were uploaded on the web server and graded via telemedicine. The authors concluded that teleophthalmology could reduce time and money in cases where a great distance between patient and doctor existed.
Finland
Lemmetty et al. presented a study on DR screening programme in Finland.43 They showed a mobile digital eye fundus camera system for screening type 2 DM eyes in the Finnish rural region of Southern Ostrobothnia. A trained diabetic nurse, who saved the pictures, and sent to hospital grading centre, took the photographs. The nurse made the first grading and the problem eyes were sent to a referral centre. The digital DR photo screening improved screen covering of patients with diabetes and helped to reduce more advanced DR needed referral to the ophthalmology hospitals.
Israel
Levy et al. described a study on DR screening in Southern Israel.44 They described a service for DR screening with the use of the Topcon mobile non-mydriatic fundus camera. A trained photographer took at least two 45° images of retina-covering macula, optic disc and superior and temporal fields. The TIFF format pictures were sent to a grading server and within 2 weeks graded by the same doctor. They concluded that mobile digital photography increased the possibility of detecting sight-threatening DR.
Germany
Kernt et al. conducted a study on DR screening comparing ETDRS seven-field stereo standard versus one ultrawide field scanning laser ophthalmoscopy (Optomap).45 They showed that the Optomap system did not need to dilate the pupil, the skills of the photographer may be not as good as the ETDRS standard and the efficiency of assessment DR is comparable. They concluded that ultrawide scanning laser ophthalmoscopy might be a promising alternative for DR screening.The authors from the same university also compared ultrawide field scanning laser ophthalmoscopy with two-field fundus photography in DR screening.46 They showed that ultrawide field ophthalmoscopy might be potentially better in DR screening, but it required further study.
Singapore
Mass screening for DR in Singapore started in 1991.47 The founders of that programme chose Polaroid non-mydriatic 45° Topcon fundus photography because of an ophthalmologist was not required to take a picture. At that time the method was relatively cheap and suitable for mass screening. Pictures were taken by a trained photographer, usually after mydriasis and graded by hospital ophthalmologist. It was the first-in-the-world nationwide programme for DR screening.
Lim et al. showed in their study review of suspected DR patients sent to tertiary referral clinic from the Nationwide Screening Programme.48 The programme is based on a one-field digital 45° image of central retina, performed annually in all patients with diabetes in Singapore. They found that one of three patients have any kind of DR and only one of nine patients has sight-threatening DR. The screening programme used a Topcon fundus camera and the pictures were graded by trained family doctors.
Taiwan
Liu et al. presented a study on DR screening in an outpatient clinic performed between 1 January 1990 till 31 December 1992.49 They used ophthalmoscopy or fluorescein angiography for DR evaluation. The authors suggested that DR screening is worthwhile.
Ireland
In 2007 in Ireland, the Diabetes Expert Advisory Group was established to develop a national, population-based DR screening programme.50 The group created a framework for DR screening: it was never implemented.
When this screening programme did not begin, in 2003 McHugh et al. presented a study for DR screening in primary care in Ireland.51 They described Diabetes in General Practice (DiGP), a community-based initiative established in 2010, involving GPs, local optometrists and ophthalmologists. They screened patients with diabetes type 1 or 2, aged 18 or more with the use of digital fundus cameras. They took two-field pictures of the retina (macula and optic disc). The pictures were graded by MDTS software and the results in electronic records were sent to GPs, who were responsible for referring patients to the ophthalmic specialists. The study was conducted from 1 January until 30 June 2011 and found 26 % patients with DR. So they concluded the need for a national DR screening programme in Ireland.
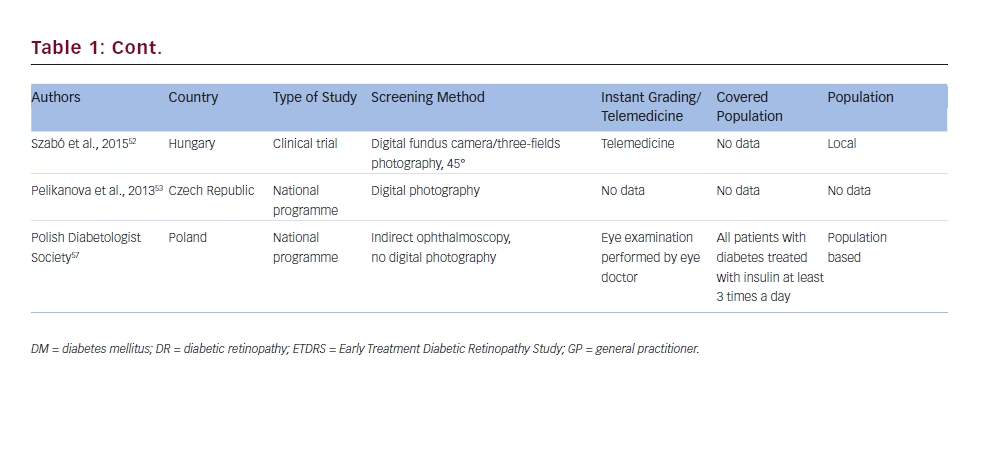
Hungary
Czech Republic
The Czech National Diabetes Programme 2012–202253 also covers DR screening with the use of photography of retina. However, currently there is not enough information on this study.Australia
It is a large country with many dispersed small towns and communities of people. This is why the DR screening is hard to carry out. Mak et al.54 and Murray et al.55 reported a DR-screening scheme in the Kimberly region of Western Australia. They described DR screening with the use of a non-mydriatic fundus camera 45°, but with Polaroid film. They did not use digital cameras because of difficulties with electricity in some places of examination. They used mobile cameras with instant grading of retinal pictures. Photographs were taken with mydriasis.
Portugal
One of the newest DR screening programmes study was reported by Dutra Medeiros et al.56 They worked in the Study Group for Diabetic Retinopathy Screening (RETINODIAB) in Lisbon and Tagus Valley area from July 2009 till October 2014. The screening programme was held in several primary healthcare units with 45° non-mydriatic fundus cameras. They took two pictures of the retina: one of the macula centre; the second of the optic disc. When problems with good quality pictures were experienced, they performed pharmacological pupil dilatation. Photographs after compression in the DICOM format were sent to the grading centre, where they were assessed according to the International Clinical Diabetic Retinopathy Scale. The study group suggested that this screening method could be an effective technique for early DR detection.
Poland
There are standards for patients with diabetes care, prepared by the Polish Diabetologist Society.57 The authors suggest that DR should be screened regularly, but there is no national programme for populationbased DR screening.
The Polish National Health Fund (NFZ) recommend diabetic eye screening (with retina examination) performed by ophthalmologists in Complex Out-Patient Specialistic Care for Diabetes Mellitus (KAOS). This service does not include national population-based DR screening.58
Discussion
There are different methods of DR screening in the world. Direct and indirect ophthalmoscopy is used, as well as different kinds of retinal photography.11–55 Conventional photography of the retina was used in early 1990 in Singapore.48 The digital photography of the eye fundus seems to be the most efficient and cost-effective. Retinal pictures are solid proof of the status of the retina and can be graded once again if needed. The gold standard for retinal diabetic photography is a seven-field stereo colour ETDRS standard,59 but this type of the fundus photography is time-consuming, expensive and requires an experienced photographer. The screening process should be: ‘economically balanced in relation to possible expenditure on medical care as a whole’8 and this condition is made by digital, non-stereo retinal photography. There are also variations of this kind of photography, from seven-field colour retinal photography recommended by the American Diabetes Association (ADA)27 and trained in Canada,25 to five-field photography30,31,34 performed in France and Spain. There are also fourfield photographs of the retina37,38 taken in Spain and three-field retinal images24,33,34 (also in France and in the US). The most-often used method is two-field pictures of the retina: performed in the UK, USA, Israel and Ireland.11,12,16–18,25,44,51 Similarly, only one picture of the retina is used in Scotland,21 Spain,36 South Africa41 and Singapore.48 A special type of onefield retinal picture in the German project is the use of one, ultrawide picture taken by Optomap,45,46 which is a interesting alternative for two-field photography. The intervals between eye examinations in the above-mentioned studies are based on the severity of the retinal changes. There are recent studies and programmes that personalise intervals of screening timing not only on retinal status but also on DM control, e.g. blood glucose control (HbA1c), blood pressure, patient gender, type of DM and duration of DM.60,61 Mehlsen et al. in Denmark and Stefansson et al. in Iceland introduced this approach for screening intervals. It allows screening to be more cost-effective through extending the length between examinations to more than 1 year for mild stages of DR. It helps also to avoid excessive retinal treatment.
Mansberger et al.29 compared telemedicine DR screening to traditional retina examination. They showed that telemedicine increased the percentage of DR screening examinations. They concluded that remote DR screening could be performed by primary care clinics.
One of the most important reasons for the use telemedicine in ophthalmology is the lack of retina specialists, even in developed countries. The use of remote assessment of retina pictures allows this problem to be managed (few doctors screen a large area). Dutra Medeiros et al. remarked on this problem in their study.56
In conclusion, it can be argued that DR screening programmes are developed all over the world, especially in developed countries. These programmes help to detect early sight-threatening DR, treat it in a timely fashion and in this way avoid expensive, advanced treatment or even prevent blindness developing among working age people. In summary, we can conclude that a good DR screening programme is simple, easy to perform, cheap, covering more than 80 % of patients, and protecting against serious complications. After a review of current DR studies, we recommend DR screening with the use of colour, two-field retina photography taken by technicians with the non-mydriatic fundus cameras, with telemedicine software and graded in centres by retina specialists.







