Corticosteroids are the best choice for treating various ocular conditions affecting the anterior and posterior segments of the eye.1 Currently, different routes are available for ocular administration of corticosteroids. Topical application to the eye is the route of choice when targeting diseases affecting the ocular surface and anterior segment, whereas posterior segment conditions may require periocular, sub-Tenon and intravitreal injections.2
The suprachoroidal space (SCS) is a unique physiological space between the sclera and choroid. It can serve as a potential space for drug delivery where the sclera and choroid have no strong attachments.3 It is important to maintain intraocular pressure (IOP) via uveoscleral outflow, which is an alternative drainage route for aqueous humour.4
Suprachoroidal injection is a promising, minimally invasive procedure for drug delivery to the posterior segment of the eye.5 Many methods have been used to access the SCS, including a microcatheter, a standard hypodermic needle, a hollow microneedle, a custom-made needle and a microinjector.
The microcatheter is manually inserted through an ab externo scleral incision to reach the SCS.6 The procedure is more invasive than other ocular methods, and it requires a skilled specialist and an operating room setting. It has the advantage of more precise targeting and visualization of the delivery area.7 Although standard hypodermic needles are less invasive, they require a high degree of skill to access the targeted area. As there is no visualization, this technique carries the risk of inadvertent injection into the vitreous or the subretinal space.8 Hollow microneedles are needles of micron dimensions that are designed to fit the length of the approximate thickness of the sclera. They have an empty space inside and can infuse the drug with accurate targeting. Microneedles are simple to use and provide a minimally invasive method to inject into the SCS.9
A needle can be customized using materials already available in the operating room, e.g. inserting a 27 gauge needle into a silicon tube and cutting the tube to expose only 1.0 mm of the 27 gauge needle. The method is easy to use and has acceptable safety outcomes.10 The suprachoroidal microinjector is applied at the pars plana into the sclera at an angle perpendicular to the injection location. The method is simple and does not require a surgical incision. The needle length is slightly longer than the scleral and conjunctival thickness at the pars plana, allowing for the penetration through the sclera without reaching the vitreous.8,11
Using a novel cannula system and monitoring the choroidal and retinal blood flow in monkeys and pigs, Olsen et al. determined that the microcannulation system can be performed in a safe and reproducible manner by using careful surgical technique.12 Poole et al. observed suprachoroidal injection of sodium hyaluronate in 14 patients and reported slight bleeding and inflammation at the site of injection, which disappeared within 3 weeks.13
Suprachoroidal routes have been evaluated by targeting the choroid, retinal pigment epithelium (RPE) and retina,9,14,15 with limited drug exposure in the anterior segment tissues (lens, iris and ciliary body).16 For example, Patel et al. found that, in suprachoroidal injections, the concentration of injected materials (fluorescein, fluorescently tagged dextrans, polymeric particles and bevacizumab) was at least 10-fold higher in the posterior segment tissues compared with the anterior segment tissues.17 The suprachoroidal route of triamcinolone acetonide administration in rabbits showed a maximum concentration of the drug in the sclera, choroid and retina, with relatively small amounts of the drug in the lens, vitreous and anterior segment.18 In preclinical studies, suprachoroidal triamcinolone acetonide achieved high levels of the drug in the retina, RPE and choroid that was detectable for more than 3 months.19 Despite the great results of corticosteroids in halting several ocular diseases and improving vision, adverse events (AEs) like steroid-induced elevation of IOP and cataract formation remain the most significant local risks following topical, sub-Tenon, intravitreal and systemic corticosteroid administration.20 More targeted drug deliveries, such as suprachoroidal delivery, may reduce side effects of corticosteroids.21
Suprachoroidal injection of triamcinolone acetonide in clinical practice
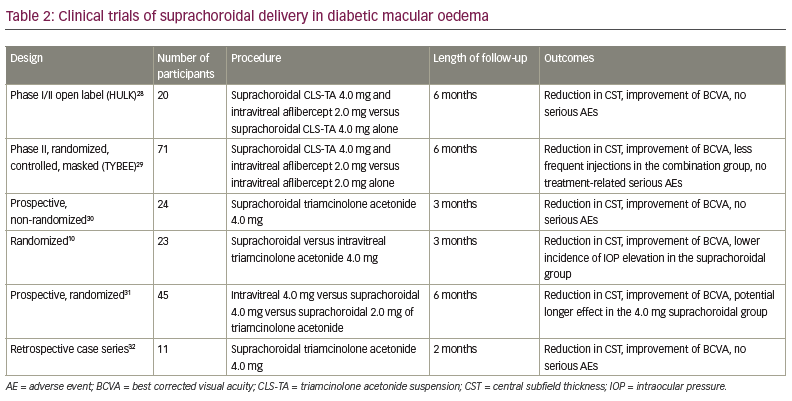
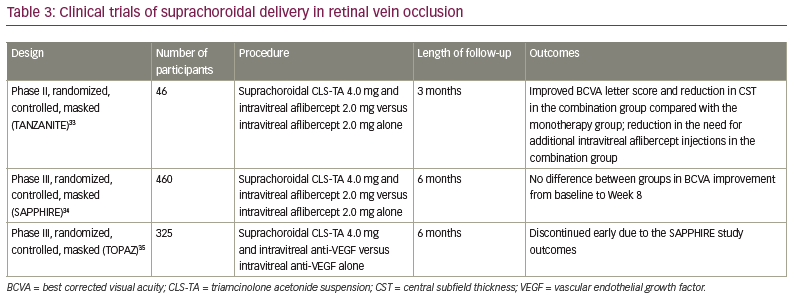
The clinical trials of suprachoroidal injection are summarized in Tables 1–3.10,22–35

Macular oedema associated with non-infectious uveitis
Suprachoroidal injection of triamcinolone acetonide was first evaluated in a phase I/II open-label clinical study in nine patients with bilateral, non-infectious anterior, intermediate or pan-uveitis.22 Patients received a single suprachoroidal injection of 4.0 mg triamcinolone acetonide in 100.0 μL in one eye and were observed for 26 weeks. Mild AEs were reported in most cases. The most common AE was eye pain, which was related to the procedure rather than the drug. One event of cataract progression lead to extraction, and no steroid-induced elevation of IOP was observed. All patients showed improvement in best corrected visual acuity (BCVA). Seven patients with macular oedema at baseline showed a reduction of retinal thickness ranging from 76.0 μm to 154.0 μm during the 26-week study. Despite the limitation of a small sample size in the study, the treatment was safe and effective in all patients.22
The randomized, controlled, masked phase II DOGWOOD study evaluated the safety and efficacy of a single suprachoroidal injection of triamcinolone acetonide suspension (CLS-TA) (4.0 mg and 0.8 mg in a 4:1 ratio) in patients with non-infectious uveitis (NIU) with macular oedema.23 Twenty-two adults were enrolled and assessed 1 and 2 months after injection. The primary efficacy endpoint was change in central subfield thickness (CST) on optical coherence tomography (OCT) from baseline. The primary endpoint was met in patients who received the 4.0 mg injection. At Month 2, 69% of patients who received the 4.0 mg injection showed ≥20% reduction in CST, with improvement of >5 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters in 65% of patients, with a mean improvement of 9.2 letters (p=0.0004). The treatment was deemed to be tolerable and safe, as no elevation of IOP or cataract development were reported.23
The phase III, double-masked, randomized PEACHTREE trial included 160 patients with macular oedema associated with NIU.24 The patient cohort was randomized 3:2 to receive a suprachoroidal injection of CLS-TA (0.1 mL in 40.0 mg/mL) or a sham treatment at Day 0, and another injection at Week 12. Rescue therapy was administered if indicated. The primary endpoint included improvement of 15 or more ETDRS letters from baseline at Week 24. The secondary endpoint was reduction in CST from baseline at Week 24 on OCT. Additional endpoints included mean change in BCVA in ETDRS letters by visit, mean change in CST by visit, and changes in signs of intraocular inflammation by visit. Patients were assessed every 4 weeks for up to 24 weeks. The primary endpoint was achieved at Week 24 in 46.9% of patients in the CLS-TA group and 15.6% in the control group (p<0.001). Improvement of BCVA from baseline was observed early at Week 4, with continuous improvements through the 24 weeks. A reduction in CST at Week 24 from baseline was greater in the CLS-TA group, about 153.0 μm compared with 18.0 μm (p<0.001) in the control group. At Week 4, 53% of patients in the CLS-TA group presented with macular oedema resolution (CST <300.0 μm) versus only 2% in the control group. The most common AE was eye pain at time of the injection. IOP elevation was seen in 11.5% of the CLS-TA group versus 15.6% in the control group. Cataract development was noted in 7.3% of CLS-TA versus 6.3% of control groups. No serious AEs related to the treatment were observed.24
The extended MAGNOLIA study evaluated the long-term safety and efficacy of CLS-TA that was administered suprachoroidally in the PEACHTREE study.25 Thirty-three patients were enrolled for an additional 24 weeks observation with follow-up visits every 6 weeks (28 of the CLS-TA group and five of the control group). The last visit of PEACHTREE was considered to be Day 0 of the MAGNOLIA study. Patients were eligible for the study if they did not need any rescue treatment during the PEACHTREE study. The primary endpoint was time to rescue therapy from the date of first study treatment administration in PEACHTREE. Additional endpoints included changes from baseline in BCVA, CST on OCT and signs of intraocular inflammation. Safety endpoints included the incidences of treatment-emergent AEs, on-study AEs and serious AEs. Eleven patients (39.3%) in the CLS-TA group received rescue therapy during the MAGNOLIA trial, whereas 14 patients (50%) did not require rescue therapy for up to 9 months after the second injection. Three patients (60%) in the control group received rescue therapy, whereas two patients (40%) did not require it. The median time to rescue therapy relative to the last injection was 257.0 days in the CLS-TA group versus 55.5 days in the control group. In the CLS-TA group, the mean increase in BCVA was 16.8 ETDRS letters at Day 0 of MAGNOLIA (p<0.001) and 12.1 ETDRS letters at the end of the study (p=0.004). The mean CST reduction was 178.1 µm at Day 0 of MAGNOLIA (p<0.001) and 174.5 µm at the end of the study (p<0.001). Four patients (14.3%) in the CLS-TA group reported IOP elevation. Cataract progression was reported in seven patients (25%) in the CLS-TA group versus one (20%) in the control group. In summary, PEACHTREE and MAGNOLIA support the administration of triamcinolone acetonide suprachoroidally as a safe and effective treatment for macular oedema associated with NIU.25
The AZALEA trial was an open-label multicentre study that evaluated the local and systemic safety of suprachoroidal injection of CLS-TA in patients with NIU.26 The study included one suprachoroidal injection of CLS-TA (0.1 mL in 40.0 mg/mL) at Day 0 and another injection at Week 12. Thirty-eight patients were enrolled in the study, and they were evaluated every 4 weeks for 24 weeks. The main safety endpoint was the incidence of treatment-emergent AEs and serious AEs in the safety population. Additional safety endpoints included frequency of vision loss, elevated IOP, cataract formation and plasma triamcinolone acetonide concentrations after treatment. No treatment-emergent AEs or serious AEs leading to study discontinuation were reported. Eye pain at the time of the procedure was seen in three patients (7.9%). Six patients (15.8%) had an IOP elevation >10.0 mmHg compared with baseline, while two patients (5.3%) had IOP >30.0 mmHg at Week 8. Four patients (10.5%) had cataract progression, but none of them needed cataract surgery. Ninety-one pharmacokinetic samples were collected during the study; 38 of them had no quantifiable triamcinolone acetonide levels. The quantifiable samples were all less than 1.0 ng/mL. Although the sample size of the study was small, AZALEA demonstrated a clinically safe method for treating NIU.26
The final study reviewed here was a prospective, non-randomized interventional study that included 30 patients who received one injection of triamcinolone acetonide (0.1 mL in 40.0 mg/mL) into the SCS.27 At the end of the 3-month study, all patients reported a reduction of more than 60% in central macular thickness (the mean central macular thickness at Day 0 was 569.6 ± 170.4 µm versus 208.2 ± 37.3 µm at Month 3, p<0.001). A functional improvement was also reported in all patients (mean BCVA at presentation 0.1415 ± 0.0694 and 0.4692 ± 0.1628 at Month 3, p<0.001). In this short-term study, none of the patients experienced elevated IOP, but five patients had changes in their lens/vitreous humour. The study reported the safety and efficacy of single suprachoroidal injection of triamcinolone acetonide in NIU over a short-term observation, and further larger studies with long-term observation are indicated.27
Diabetic macular oedema
The open-label, phase I/II HULK study evaluated suprachoroidal CLS-TA as monotherapy or in combination with intravitreal aflibercept in diabetic macular oedema (DMO).28 Twenty patients were enrolled: 10 eyes defined as the treatment-naïve group received a suprachoroidal injection of CLS-TA (4.0 mg/100 µL) and intravitreal injection of aflibercept (2.0 mg/0.05 mL), and 10 eyes that had previously been treated with either anti-vascular endothelial growth factors (VEGF) or intravitreal corticosteroids received CLS-TA suprachoroidally. Patients were observed for 6 months. Improvement in BCVA from baseline occurred in all patients at Month 6 (mean increase of 8.5 versus 1.1 ETDRS letters in the combination and monotherapy groups, respectively). Overall, 89% of patients had a reduction in CST of more than 50%. Two patients in the monotherapy group had an IOP elevation >10.0 mmHg. Cataract progression was reported in three patients.28
The randomized, controlled, masked, phase II TYBEE study included 71 patients with DMO that were naïve to treatment.29 The study participants were randomized to active and control groups and observed for 24 weeks. The active group (36 eyes) received CLS-TA (4.0 mg/100.0 µL) and aflibercept (2.0 mg/0.05 mL) at baseline and Week 12, while the control group (35 eyes) received aflibercept at baseline and at Weeks 4, 8 and 12. Both groups showed improvement in BCVA with similar visual benefits at Week 24 (mean increase of 12.3 versus 13.5 EDTRS letters [p=0.664] in the active and control groups, respectively). Reduction in CST was 212.1 μm in the active group versus 178.6 μm in the control group (p=0.089). No treatment-related serious AEs were reported over the 24 weeks of follow-up. Elevation of IOP was reported in three (8.3%) patients in the active group and one (2.9%) patient in the control group. Cataract progression occurred in two (5.5%) patients in the active group and one (2.9%) patient in the control group. The results show similar visual benefit in both groups, with modest anatomic benefit and fewer intravitreal injections required in the active group.29
Another prospective, non-randomized interventional study evaluated suprachoroidal triamcinolone acetonide (0.1 mL in 40.0 mg/mL) in resistant DMO.30 The study included 24 patients observed over a 3-month period. DMO was defined as resistant when it failed to respond to three anti-VEGF injections spaced at 1 month apart. Mean BCVA improved from 0.8 ± 0.24 ETDRS letters at baseline to 0.45 ± 0.27 EDTRS letters at Month 3 (p<0.05). The mean CST reduced from 636.5 ± 200.1 µm at baseline to 302.7 ± 66.9 µm at Month 3 (p<0.00001). There was no difference in IOP pre- and post-injection. One patient had an elevation of IOP from 19.0 mmHg to 24.0 mmHg at Month 1. This patient received topical antiglaucoma therapy and the IOP returned to 16.0 mmHg by the 3-month follow-up. No other AEs were reported during the study. Despite the limitations of a small sample size and short-term follow-up, the study demonstrated safe and effective therapy in all patients.30
Abdelshafy Tabl et al. reported a randomized clinical trial that compared the efficacy and safety of suprachoroidal and intravitreal injection of triamcinolone acetonide in refractory DMO due to epiretinal membrane.10 Twenty-three patients with pseudophakia were randomized to suprachoroidal (13 eyes) or intravitreal (10 eyes) triamcinolone acetonide (0.1 mL in 40.0 mg/mL) and evaluated for 3 months. Both groups reported improvement in mean BCVA; there was no significant difference between groups regarding BCVA at baseline and Months 1 and 3 (mean central foveal thickness was 323.0 µm in the suprachoroidal group versus 385.0 µm in the intravitreal group, p=0.028). The IOP at Month 1 and Month 3 was significantly higher in the intravitreal group (12.0 mmHg in the suprachoroidal group versus 15.0 mmHg in the intravitreal group at Month 1, p=0.011; 14.0 mmHg versus 18.0 mmHg, respectively, at Month 3, p=0.028). In this short-term study, both groups reported visual and anatomical improvement with lower incidence of IOP elevation in the suprachoroidal group.10
Another prospective randomized study compared suprachoroidal and intravitreal injection of triamcinolone acetonide in DMO.31 The study included 45 eyes of 32 patients who were divided into three groups (15 eyes in each group); group I received 4.0 mg/0.1 mL intravitreal triamcinolone acetonide group II received 4.0 mg/0.1 mL suprachoroidal triamcinolone acetonide, and group III received 2.0 mg/0.1 mL suprachoroidal triamcinolone acetonide. Patients were observed for 6 months. BCVA improved in all groups at Month 1 and Month 3 compared with baseline, with no significant differences between groups. At Month 6, BCVA decreased to near-baseline values, except in group II where vision remained relatively stable. All groups showed a significant reduction in central macular thickness at Month 1 (p=0.005, 0.008 and 0.001 in groups I, II and III, respectively). It started to increase again at Month 3; the reduction was not statistically significant compared with baseline, except in group II (149.80 ± 106.57 μm, p=0.013). The reduction returned close to the baseline values at Month 6, except in group II that showed the most reduction in central macular thickness over the 6-month follow-up period (60.16 μm from baseline). IOP elevation was reported in one (6.7%), two (13.3%) and one eye (6.7%) in groups I, II and III, respectively. All patients were successfully treated with topical antiglaucoma therapy. Cataract progression was reported in three eyes per group. This study demonstrated the safety and efficacy of both suprachoroidal and intravitreal injections of triamcinolone acetonide in DMO, with a potential longer effect in suprachoroidal delivery.31
Recently, a retrospective case series evaluated the efficacy and safety of suprachoroidal 4.0 mg/0.1 mL triamcinolone acetonide in DMO post-pars plana vitrectomy (PPV).32 Eleven eyes of ten patients were observed during the 8-week follow-up period. Mean BCVA improved from 0.75 ± 0.40 log minimum angle of resolution (MAR) at baseline to 0.40 ± 0.33 logMAR at 8 weeks (p=0.003). Mean central macular thickness reduced from 456.45 ± 113.42 μm at baseline to 247.63 ± 53.40 μm at 8 weeks (p=0.003). No IOP elevation or cataract progression in the single treated phakic eye was observed during the 8-week follow-up period. In this short-term study, suprachoroidal injection of triamcinolone acetonide showed promising results in improving visual and anatomical outcomes in DMO post-PPV, with no serious AEs.32
Retinal vein occlusion
The phase II, randomized, masked TANZANITE clinical trial evaluated the combination of suprachoroidal CLS-TA (4.0 mg/100.0 µL) and intravitreal aflibercept (2.0 mg/0.05 mL) versus aflibercept (2.0 mg/0.05 mL) alone in patients with macular oedema due to retinal vein occlusion (RVO).33 Forty-six patients were observed for 3 months. Patients in each study group received as-needed aflibercept at Months 1, 2 and 3. The primary endpoint was the number of protocol-required aflibercept re-treatments during the 3-month period. Secondary endpoints included mean improvement from baseline in BCVA, CST and the percentage of participants with CST ≤310.0 μm. The primary endpoint was achieved, as the number of aflibercept re-treatments was significantly lower in the combination group compared with the aflibercept group (23 versus 9, respectively, 61% reduction, p=0.013) and the percentage of participants requiring no re-treatments was higher in the combination group (78% versus 30%, p=0.003). The mean improvement from baseline in BCVA was significantly higher in the combination group compared with the aflibercept group (18.9 versus 11.3 ETDRS letters at Month 3, p=0.09). The mean CST in the combination group decreased from 731.1 μm at baseline to 284.7 μm at Month 1, and was stable at Month 2 (272.4 μm) and Month 3 (285.4 μm). The mean CST in the aflibercept group decreased from 727.5 μm at baseline to 322.8 μm at Month 1, and increased at Month 2 (383.4 μm) and Month 3 (384.6 μm). Higher percentages of oedema resolution (CST ≤310.0 μm) occurred in the combination group (78.3% versus 47.8%) at Month 3. Four patients in the combination group had IOP elevation that was treated with topical antiglaucoma therapy. The study showed the safety and efficacy of suprachoroidal CLS-TA in RVO, which reduced the need for additional intravitreal aflibercept injections during the 3-month follow-up period.33
The phase III SAPPHIRE study compared suprachoroidal injection of CLS-TA with intravitreal aflibercept versus intravitreal aflibercept alone in RVO in 460 eyes over 12 months.34 Approximately 50.0% of patients in both groups reported improvement in BCVA ≥15 ETDRS letters at 8 weeks. There was no additional benefit of the combination therapy, therefore, the study was discontinued.34
The phase III TOPAZ study had a similar design to the SAPPHIRE study, using either aflibercept (0.5 mg/0.05 mL) or bevacizumab (1.25 mg/0.05 mL) intravitreally instead of aflibercept alone in both groups. The trial was discontinued early due to the SAPPHIRE study outcomes.35
Pseudophakic cystoid macular oedema
Suprachoroidal triamcinolone acetonide is also used in patients with pseudophakic cystoid macular oedema (PCMO). Oli and Waikar published a case series of three patients with non-resolving PCMO who each received a single suprachoroidal injection of triamcinolone acetonide (4.0 mg/0.1 mL) and were observed for 12 weeks.36 The mean BCVA improved from 1.0 logMAR at baseline to 0.3 logMAR at Week 4 and was stable over 6 months. The mean central macular thickness reduced from 473.5 μm at baseline to 287.0 μm at Week 12. IOP remained within normal limits and no serious AEs were reported.36
A recent case study reported results of a patient with PCMO that was treated with one injection of triamcinolone acetonide (4.0 mg/0.1 mL) suprachoroidally and observed for 24 weeks.37 BCVA improved from 20/60 at baseline to 20/30 at Week 24. Central macular thickness reduced from 586.0 μm at baseline to 257.0 μm at Week 24. No IOP elevation was reported. Although the treatment was safe and effective, larger randomized studies are indicated to evaluate persistence and safety of the therapy in the long term.37
Solar retinopathy
A case report was published showing the effect of triamcinolone acetonide in solar retinopathy in a patient with low vision. A single injection of triamcinolone acetonide (4.0 mg/0.1 mL) was administered suprachoroidally. After 4 months of follow-up, BCVA improved from 0.1 (Snellen chart) to 1.0, with an anatomical improvement in the ellipsoid zone layer on OCT. IOP elevation was noted at Week 7 and was controlled with topical antiglaucoma therapy. No serious AEs were reported.38
Serous choroidal detachment
A single-centre, prospective pilot study evaluated the efficacy of preoperative suprachoroidal triamcinolone acetonide on the resolution of serous choroidal detachment (CD) associated with rhegmatogenous retinal detachment.39 Ten eyes were involved in the study. More than 50% reduction in cumulative mean height of CD was seen in five eyes by Day 3 and in two eyes by Day 5. Three eyes did not respond to the treatment. IOP was raised in one eye at Day 30 post-PPV and was controlled with topical antiglaucoma therapy. No other complications were reported after the injection. Although the study was limited by a small sample size, the procedure was safe and effective in achieving rapid resolution of serous CD.39
Conclusions
Suprachoroidal delivery has become a new alternative route for treating various ocular conditions. The aim is to achieve a higher drug concentration in the posterior segment targeting the choroid, RPE and retina, which may enhance efficacy of the treatment, with reduced exposure in the anterior segment, thus decreasing side effects such as IOP elevation and cataract progression. Preclinical studies of suprachoroidal triamcinolone acetonide achieved high levels of the drug in the retina, RPE and choroid that was detectable for more than 3 months.19 Clinical trials of suprachoroidal triamcinolone acetonide support this unique treatment as a potential route for drug delivery. The treatment improved visual acuity and reduced macular oedema in patients with NIU, DMO, RVO or PCMO.24,30,33,36 In the MAGNOLIA study, the efficacy and safety of CLS-TA in NIU were maintained in 50.0% of patients for up to 9 months after the second injection.25 The suprachoroidal route has demonstrated a potential longer effect in DMO for up to 6 months compared with the intravitreal route, where the central macular thickness started to increase after 3 months and the reduction was not statistically significant compared with baseline.31 In RVO, patients who received the combination therapy of triamcinolone acetonide and aflibercept reported significantly better improvement in BCVA with stable CST reduction during the 3-month follow-up period compared with aflibercept alone.33 In DMO and RVO, patients who received suprachoroidal triamcinolone acetonide required fewer intravitreal aflibercept injections compared with those who received aflibercept alone.29,33
The treatment was generally well tolerated with acceptable safety outcomes in all patients. Eye pain at the time of the procedure was the most common AE.21 The suprachoroidal route has shown a lower incidence of IOP elevation than the intravitreal route.10 These data are supported by the recent POINT40 study that reported a 26.0% rate of IOP elevation (IOP increase >10.0 mmHg) with the use of intravitreal triamcinolone acetonide compared with a 14.3% rate observed at the conclusion of the MAGNOLIA study.25 Patients who reported IOP elevation were treated successfully with topical antiglaucoma therapy. Across all studies, no infectious complications or serious AEs related to the treatment were observed. Taking into consideration that the incidence of endophthalmitis post-intravitreal injection ranges from 0.029% to 0.056%,41–43 larger studies are required to evaluate the safety of the suprachoroidal route.
In conclusion, although the treatment is tolerable and effective, further larger-scale studies are needed to compare the long-term safety and efficacy of suprachoroidal triamcinolone acetonide with different ocular drug routes.







