Various options for the prevention of pseudophakic cystoid macular oedema (PCMO) have been offered. Nonsteroidal anti-inflammatory drugs (NSAIDs) seem to be beneficial in preventing postoperative inflammation; however, there is lack of evidence for long-term benefit after cataract surgery. What is more, topical NSAID preparations are difficult to compare, as studies differ in inclusion criteria, patient characteristics, prescription and duration of treatment. There is ongoing discussion whether NSAIDs and corticosteroids have an additive or synergistic effect. In this article, we provide an up-to-date review of the different strategies used for the prevention of PCMO. Pubmed and Cochrane Library were used to search for studies published up to 1 May 2019. We used key search terms, such as ‘pseudophakic cystic macular edema’, ‘post-operative inflammation’, ‘cataract surgery’ and ‘nonsteroidal anti-inflammatory drugs’. Reference lists of identified studies were also searched. We included only articles that had abstracts in English language. Relevant information from selected textbooks was also included. We analysed and compared studies to create an overview of PCMO with focus on the prevention.
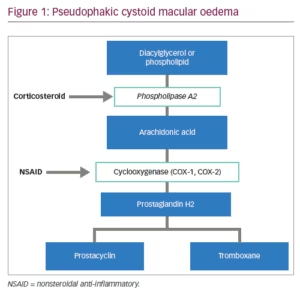
Cataract is one of the leading causes of severe vision loss.1 Senile or age-related cataract is most common type so the prevalence rate increases as the population ages.2 It is possible to restore vision in patients with cataracts by removing the opacification of the lens. Cataract surgery is a widely used procedure considered to be safe and effective. Due to mechanical damage implicated in the surgery and reaction to the artificial intraocular lens and other non-infectious substances entering the eye during surgery, various level of inflammation may occur.3 Surgical trauma causes the release of prostaglandins (Figure 1), which affect the eye structures and mediate inflammatory reactions. Prostaglandins increase vascular permeability and dilation, and white blood cell reaction and migration. Postoperative inflammation is quite a common reaction, although untreated might cause complications – formation of the synechiae, corneal oedema, elevation of intraocular pressure (IOP), cystoid macular oedema.4 Patient discomfort, pain, delayed recovery and suboptimal visual outcome after cataract surgery can be caused by uncontrolled inflammation.5 Inflammation of the eye and excreted prostaglandins can also cause smooth muscle contraction in the iris which leads to miosis during surgery.6
The exact pathogenesis of PCMO remains unclear, although it is thought to be multifactorial. It is known that surgical manipulation in the anterior chamber may cause the release of arachidonic acid from uveal tissue.7 Arachidonic acid is metabolised into prostaglandins, which are inflammatory mediators that cause swelling of the fovea by increasing vascular permeability and fluid accumulation due to the breakdown of the blood–aqueous and blood–retinal barriers.8,9 PCMO is thought to be a common cause of unsatisfactory functional outcome with a prevalence ranging from 0.10–2.35%.9 Incidence of PCMO depends on how cystoid macular oedema is defined.6 In patients who are not diabetic and without ocular risk factors, such as iris trauma and vitreous loss, the incidence is 1.17%.10 Not all cases of PCMO experience functional impairment, and these are called sub-clinical PCMO. Conversely those eyes with visual impairment are designated clinically significant.
A decrease in visual acuity is not always present, but fluorescein angiography can show evidence of macular oedema. The presence of PCMO can be determined by using optical coherence tomography (OCT) – diagnostic tool that allows objective measurement of changes in retina volume. The incidence of PCMO defined by OCT after uncomplicated phacoemulsification in a recent study was found to be 2.72%.11 Usually mild subclinical episodes of PCMO resolve spontaneously.12 Lobo et al. have shown that macular oedema reaches a maximum at 6 weeks, after which, most cases led to recovery; although seven eyes (22%) still had macular oedema in 30 weeks.13 Henderson et al. reported that PCMO in patients after cataract surgery resolved in 249.0 ± 2.8 days with no treatment, and that patients treated with combined NSAIDs and corticosteroids had significantly shorter resolution times of 82.6 ± 56.9 days.14 Most reports favour treatment for cases of clinically significant PCMO to prevent irreversible damage to the macula.6
Pseudophakic cystoid macular oedema risk factors
PCMO may occur in healthy eyes without risk factors after uncomplicated cataract surgery, although it is more often in contralateral PCMO, diabetes mellitus, uveitis, retinal vein occlusion, retinal degeneration, macular degeneration, radiation retinopathy, epiretinal membranes, choroidal tumours and aging.10,15–17 Patients with diabetes, even without diabetic retinopathy, have increased risk in developing PCMO after cataract surgery, as poor glycaemic control is associated with a risk of postoperative macular edema.18 Accumulation of inflammatory cytokine concentrations in ocular tissues may contribute to early neuronal cell death in the retina.19 Hyperglycaemia increases concentration of circulating cytokines due to oxidation mechanisms.20 Raised levels of inflammatory and proliferative factors may contribute to pathogenesis of proliferative diabetic retinopathy (PDR) and even correlate with the severity of PDR (interleukin 6 [IL-6], vascular endothelial growth factor [VEGF], transforming growth factor [TGFb-1]).18,21 Risk of PCMO was found to be proportionate to the severity of diabetic retinopathy.10 In a multicentre database study was conducted by Denniston et al. which included patients undergoing cataract surgery with diabetes and no history of diabetic macular oedema. In the first year after surgery risk of developing diabetic macular oedema which required treatment was 1.0% for patients with no diabetic retinopathy preoperatively, 5.4% for patients with mild non-proliferative diabetic retinopathy (NPDR), 10.0% for moderate, and 13.1% for severe NPDR.22 In a study conducted by Yang et al., risk factors for PCMO after cataract surgery were duration, severity and type of diabetes; hardness of the lens; and glycated haemoglobin (HbA1c) levels.23 Patient allergy and atopy have not been found to increase the risk of PCMO.24
Nonsteroidal anti-inflammatory drugs
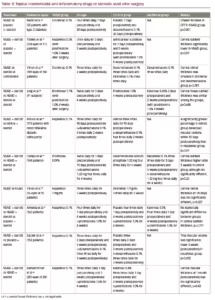
NSAIDs are chemical compounds, which inhibit cyclooxygenase (COX) enzymes and therefore inhibit the synthesis of prostaglandins.4 The US Food and Drug Administration (FDA) approved the following topical NSAIDs: nepafenac, bromfenac, ketorolac, diclofenac and flurbiprofen (Table 1). Indications for use of them are inflammation and pain associated with cataract surgery, corneal refractive surgery, inhibition of intraoperative miosis and seasonal allergic conjunctivitis.25 NSAIDs are not FDA indicated for the treatment of PCMO; however, they may be beneficial in increasing the speed of visual recovery in the first several weeks after cataract surgery.12 Topical NSAIDs may cause some side effects such as transient burning, punctate keratitis and corneal epithelial defects.4,26 NSAIDs may cause cytotoxic reactions which can lead to significant corneal alterations such as corneal melts.27

Diclofenac
Diclofenac sodium 0.1% is a derivative of phenyl acetic acid and one of the most commonly used NSAIDs. In randomised controlled trial, Medic´ et al. showed significantly lower IL-12 levels in the aqueous humour of patients with diabetic retinopathy using diclofenac 0.1% four times a day for 7 days, compared to placebo. The same study compared central foveal thickness 30 days after surgery in two groups treated with either diclofenac 0.1% or dexamethasone 0.1%, and reported lower incidence of postoperative macular oedema in the NSAID group.27 A combination of cyclodextrin (cyclic oligosaccharide) with diclofenac may lower ocular toxicity and enhance corneal tolerability.28
Nepafenac
Nepafenac is a potent prodrug which is converted to its active metabolite, amfenac, by intraocular hydrolases.29 Amfenac suppresses prostaglandin synthesis by inhibiting COX-1 and COX-2. In study conducted by Walters et al., amfenac showed greater potency at COX-2 inhibition than ketorolac 0.4% and bromfenac 0.09%.30 Nepafenac 0.1% is also considered to be safe on the ocular surface after surgery. In a prospective randomised trial, postoperative corneal staining was compared when using nepafenac 0.1% or diclofenac 0.1% beginning 3 hours before surgery and three times a day for 4 weeks postoperatively. Patients in the diclofenac group had significantly higher corneal staining scores 4 weeks postoperatively.31 Tzelikis et al. reported nepafenac 0.3% once daily, starting 2 days before surgery and 5 weeks after, was effective in reducing macular thickness compared to placebo, although without a difference in visual acuity (all patients received prednisolone acetate for 5 weeks postoperatively).32
Bromfenac
Bromfenac is an NSAID which is available in different concentrations: 0.07%, 0.075% and 0.09%. It is used to treat postoperative inflammation and ocular pain after cataract surgery, although it is not indicated for the prevention or treatment of PCMO.33 Bromfenac 0.09% has good ocular penetration properties and has been found to maintain stable concentration in the aqueous humour up to 12 hours after instillation.34 Bromfenac 0.07% and 0.09% ocular distribution were compared in a 12-hour study with rabbits, and no significant difference in ocular tissue between concentrations was found.35 Bromfenac 0.075% (BromSite®; Sun Ophthalmics, Princeton New Jersey, USA) is relatively new compound formulated with the DuraSite® delivery system (Sun Ophthalmics), a mucoadhesive matrix contributing to longer ocular surface dwelling time.25 Sheppard et al. conducted an in vivo study with rabbits, comparing the pharmacokinetics of bromfenac 0.075%, bromfenac 0.7% and nepafenac/amfenac 0.3% ophthalmic solutions. This study showed similar ocular distribution patterns in both the anterior and posterior segments, although bromfenac 0.075% (BromSite) showed significantly higher concentrations in ocular tissues.25 In an ex vivo study performed in human aqueous humour, bromfenac concentration was significantly higher in subjects receiving DuraSite containing bromfenac 0.075% than bromfenac 0.09% after 3 days of dosing.36
In several studies, bromfenac was shown to be quite well tolerated. In a study conducted by Chinchurreta Capote et al., none of the patients complained of eye stickiness when using bromfenac compared to 13.8% of patients in nepafenac group and 10.3% of patients treated with diclofenac sodium. This observational comparative study using post-surgical satisfaction questionnaire showed that bromfenac is well tolerated; moreover, bromfenac was found to be more effective than diclofenac and nepafenac in reducing PCMO after phacoemulsification.37 In another randomised controlled study, bromfenac 0.09% was also found to be better tolerated than diclofenac 0.1%; although diclofenac appeared to be more effective in reducing postoperative inflammation in terms of anterior chamber laser flare-cell photometry and central foveal thickness.38
Ketorolac
Ketorolac tromethamine is a NSAID, usually used to manage pain, including during eye surgery. Ketorolac tromethamine 0.5% has been shown to be equally effective as diclofenac sodium 0.1% in reducing the severity and duration of PCMO after cataract surgery.39 Ketorolac 0.045% ophthalmic solution containing carboxymethylcellulose was developed relatively recently. Attar et al. reported that formulation of ketorolac 0.045%, pH 6.8, increased the bioavailability in aqueous humour and iris-ciliary body more so than ketorolac 0.4%, pH 7.4.40 Waterbury et al. reported higher concentrations of ketorolac 0.045% in animal ocular tissues than bromfenac 0.09%.41
Corticosteroids
Corticosteroids act as phospholipase-A2 inhibitors and prevent the release of arachidonic acid which can be metabolised to leukotrienes and prostaglandins. (Table 2)42 This class of drugs also prevent epithelial adhesion, chemotaxis, phagocytosis.12 Adverse effects of topical corticosteroids use include IOP elevation, impaired wound healing, infection and development of cataracts.43

Dexamethasone
Dexamethasone eye drops may be applied topically after cataract surgery. Dexamethasone disodium phosphate eye drops have been found to have low penetration to aqueous humour and vitreous compared to other administration routes (peribulbar or subconjunctival injection).44 Dexamethasone disodium phosphate suspension gives three-times higher concentration in the aqueous humour when applied topically compared to dexamethasone solution.45 In a randomised, double-blind, prospective study, Ylinen et al. showed that dexamethasone monotherapy (1 mg/mL three times a day for 3 weeks) after cataract surgery had lower effect compared to diclofenac (1 mg/mL three times a day for 3 weeks) or dexamethasone and diclofenac.46 No significant difference in preventing PCMO was found when comparing postoperative use of prednisolone acetate to dexamethasone phosphate.47 Klamann et al. showed that an intravitreal 0.7 mg dexamethasone implant may be effective in postoperative macular oedema treatment.48
Prednisolone
Prednisolone acetate 1.0% is one of the most frequently used corticosteroids after cataract surgery. Prednisolone is a synthetic analogue of the glucocorticoid hydrocortisone. It has good ocular penetration characteristics and is relatively the most effective anti-inflammatory agent for anterior segment ocular inflammation.49
Difluprednate
Difluprednate or difluoroprednisolone butyrate acetate are modifications of prednisolone to increase its potency – affinity for the glucocorticoid receptor and enhance tissue penetration. It was approved by FDA in 2008,50 and is available in 0.05% ophthalmic solution. Garg et al. reported 0.05 difluprednate used six times a day for 4 weeks compared to prednisolone acetate 1.0% six times a day for 4 weeks is equally
effective in treatment of postoperative inflammation.51 In a retrospective study, Tijunelis et al. reported no significant difference in IOP change between two groups of patients using difluprednate twice a day for 30 days and prednisolone acetate four times a day for 30 days after cataract surgery.50
Triamcinolone acetonide
Subconjunctival triamcinolone acetonide may provide a prolonged anti-inflammatory effect after cataract surgery. Lindholm et al. reported a good effect on postoperative central retinal thickness and aqueous flare of 20 mg (0.5 mL; 40 mg/mL) with a perioperative subconjunctival injection of triamcinolone acetonide after cataract surgery, compared to daily dexamethasone 1 mg/mL three times a day for 3 weeks. No serious adverse events were observed in 90-day period.52 Yüksel et al. compared 40 mg sub-Tenon triamcinolone acetonide injection to twice-daily topical nepafenac 0.1% and found that both treatment modalities are effective; although nepafenac was more efficacious in terms of visual gain.53 In a randomised controlled trial, PREMED Study Report 2, subconjunctival injection of 40 mg triamcinolone acetonide was used as additional treatment for diabetic patients undergoing phacoemulsification cataract surgery. Macular thickness and macular volume were found to be lower at weeks 6 and 12, postoperatively, in patients who received triamcinolone than patients who did not.54
Prevention of pseudophakic cystoid macular oedema
Due to spontaneous resolution of most uncomplicated PCMO cases, practice patterns of PCMO prevention differ. There is an opinion that prophylaxis of PCMO is unnecessary if patients have no risk factors.55 Cataract surgery guidelines from the American Academy of Ophthalmology (AAO) recommend using NSAIDs only in high-risk cases, such as diabetic retinopathy.56 The National Institute for Health and Care Excellence (NICE) recommends to consider using a combination of topical steroids and NSAIDs after cataract surgery for people with increased risk of PCMO, for example, patients with diabetes or uveitis. Topical steroids and/or NSAIDs may be offered after cataract surgery to prevent inflammation and PCMO.57 The Canadian Ophthalmology Society recommends using topical steroids, NSAIDs or both in all intraocular surgery.58
Inflammation after cataract surgery may be controlled using topical NSAIDs or steroids (Table 3). Kessel et al. performed a systematic literature review and found that topical NSAIDs are more effective than topical steroids, and therefore recommend using them to prevent PCMO after cataract surgery.8 Although, this review was criticised for not discussing studies in which no difference in macular volume was found when comparing NSAIDs and placebo, and not including existing recommendations for the prevention of postoperative inflammation and PCMO.59 Moreover, a Cochrane systematic review has shown that value of using NSAIDs as an alternative to, or in combination with, topical steroids to prevent vision loss after cataract surgery, is uncertain.60
Almeida et al. evaluated the efficacy of ketorolac 0.5% versus nepafenac 0.1% on macular volume 1 month after cataract surgery. Patients were divided in three groups and received ketorolac 0.5%, nepafenac 0.1% or placebo. Patients were instructed to instil one drop four times a day, 1 day preoperatively and for 4 weeks postoperatively. All patients received prednisolone 1.0% for 4 weeks. No significant difference in central subfield thickness postoperatively was found between the groups.61
Ramakrishnan et al. compared the effects of 0.1% nepafenac and 0.4% ketorolac on central macular thickness after cataract surgery. Both groups received prednisolone 1.0% one month postoperatively and NSAID drops three times a day 1 day preoperatively and 30 days postoperatively. Central macular thickness 1 month postoperatively was not significant differently.62
There are studies showing the superior effect of NSAIDs in combination with steroids than steroids alone. Zaczek et al. compared two groups of patients, one received nepafenac 0.1% three times a day starting 2 days preoperatively and continued 3 weeks after and dexamethasone 0.1% three times a day for 3 weeks after cataract surgery, and other group received dexamethasone 0.1% alone. The combination of NSAID and steroid was found to have greater effect on reducing subclinical macular swelling than steroid alone.63
Jung et al. reported a beneficial effect of bromfenac 0.1% (one drop twice daily starting 3 days before cataract surgery and for 4 weeks postoperatively) or ketorolac 0.45% (one drop twice daily starting 1 day before surgery and continuing 2 weeks postoperatively) compared to prednisolone alone after cataract surgery in reducing postoperative inflammation and macular changes. All patients in the three groups received topical gatifloxacin 0.3% and prednisolone acetate 1.0% for 4 weeks postoperatively.64 In another study conducted by Pollack et al. patients were randomised to two groups, the first group using nepafenac 0.1% three times daily starting 1 day before surgery and 90 days after; and the second group using vehicle. Both groups of patients received dexamethasone 0.1% four times daily for 2 weeks postoperatively. The NSAID–steroid combination group showed a significantly lower percentage of eyes developing macular oedema within 90 days after cataract surgery.65 Campa et al. found that bromfenac 0.09% (one drop two times a day for 3 days before surgery and 15 days after) or nepafenac 0.01% (one drop three times a day for 3 days before surgery and 4 weeks after) usage with dexamethasone sodium phosphate 1.32 mg and netilmicin sulphate 4.55 mg (four times daily for 4 weeks after surgery) are associated with reduced incidence of PCMO than using dexamethasone alone.66 Toyos compared two groups of patients undergoing phacoemulsification using only once-daily bromfenac 0.07% or nepafenac 0.3% solutions and found no significant difference in terms of retinal thickness increase.67
Randomised controlled clinical trial PREMED (the prevention of macular oedema after cataract surgery) compared different prevention methods in patients without (PREMED Study Report 1) and with (PREMED Study report 2) diabetes across 12 European study centers. Study Report 1 compared NSAID, corticosteroids and their combination in the prevention of PCMO in nondiabetic patients after cataract surgery. There were three groups of patients: the first one received bromfenac 0.09% twice daily 2 days preoperatively and 2 weeks postoperatively, second group received dexamethasone 0.1% 4 times daily 2 days preoperatively and 2 weeks postoperatively, third group used combination of these two drugs. Patients underwent ophthalmologic examination at baseline and had follow-up visits at 6 and 12 weeks postoperatively. The study results showed that patients who received combination of bromfenac 0.09% and dexamethasone 0.1% had lowest postoperative macular thickness and the risk of developing clinically significant PCMO was lower than in patients treated only with bromfenac or dexamethasone. Visual acuity between the groups were not significantly different at 6 and 12 weeks postoperatively. Although PCMO incidence, which developed at 6 weeks postoperatively in most cases, was significantly lowest in combination group.68
In PREMED Study Report 2, all patients who underwent phacoemulsification received treatment with bromfenac and dexamethasone eyedrops. Patients were allocated into four groups according to the additional treatment they received. The first group were prescribed subconjunctival injection of triamcinolone acetonide, the second group received intravitreal injection of bevacizumab, the third group received both medications, and the fourth group got no additional treatment.57 Almost all patients included in the study were diagnosed with diabetes mellitus and most of them had no signs of diabetic retinopathy at the time of surgery. An extensive ophthalmologic examination was performed on the study eye, including subjective refraction, corrected distance visual acuity, slit lamp examination, tonometry, fundoscopic examination and macular thickness measurements using OCT. Patients had follow-up visits 6 and 12 weeks postoperatively and underwent ophthalmologic examination. The results of this trial show that patients who had subconjunctival injection of triamcinolone acetate postoperatively had lower mean central subfield macular thickness than patients who had not had triamcinolone acetate. Additionally, none of patients in this treatment group developed cystoid macular oedema. Although, IOP was significantly higher in patients who received the subconjunctival injection of triamcinolone acetate than patients in other groups. However, based on the results of the PREMED study, triamcinolone acetate injections are not recommended for all diabetic patients undergoing cataract surgery considering the low overall incidence of postoperative PCMO and increased IOP.54
None of these studies demonstrate a significant effect on visual acuity over longer periods (>3 months) after cataract surgery. Kim et al. performed literature review on the effectiveness of topical NSAIDs in preventing PCMO. They concluded that there is a lack of level 1 evidence to support the long-term efficacy of NSAIDs in preventing vision loss for >3 months after surgery; however, NSAIDs may improve the speed of visual recovery postoperatively.12 Pollack et al. analysed the change in best corrected visual acuity from baseline to day 90 after cataract surgery and found that the change was greater in the nepafenac group than in the vehicle group, although it was not statistically significant.65
There is an ongoing debate about cost-benefit ratio regarding prevention of PCMO.4 PCMO treatment usually involves more invasive methods, which may cost more than prevention.69 Schmier et al. found that patients who are treated for PCMO in the USA have higher Medicare claims than patients who do not develop PCMO. Of 78,949 patients who underwent cataract surgery, 2.54% (n=2,003) were diagnosed with PCMO. Ophthalmic charges were significantly higher for patients who developed PCMO (US $10,410 versus $5,950). Targeting interventions for high-risk patients are likely to be more cost-effective.70
Conclusions
Inflammation after cataract surgery remains an important cause of postoperative complications, such as intraoperative miosis, anterior uveitis, ocular hypertension or PCMO, even when using modern surgery methods. PCMO usually resolves spontaneously, although may cause a decrease in visual acuity and damage the macula when untreated. Postoperative inflammation of the eye can be relieved by steroids and/or NSAIDs. These two classes of drugs have overlapping mechanisms of action. It is not yet verified if the interaction between these drugs is synergistic or additive. Based on the present accumulation of evidence the combination of steroids and NSAIDs in the perioperative period lead to benefits in visual acuity during 12 weeks after standard cataract surgery; however, this effect disappears after this period. On the other hand, combination therapy brings clear benefits in PCMO prevention in high-risk cataract cases in both short-term and long-term follow ups.







