Reproxalap is a reactive aldehyde species (RASP) inhibitor believed to mitigate inflammatory conditions such as dry eye disease (DED), allergic conjunctivitis and uveitis.1 RASP are pro-inflammatory molecules that covalently bind to thiol and amino groups expressed on various receptors and kinases. This binding activates the pro-inflammatory signalling cascade, which involves the activation of inflammatory cytokines leading to inflammation and pro–histaminic factors leading to an allergic response.1 Malondialdehyde (MDA) is a well-characterized RASP associated with autoimmune diseases, allergic conjunctivitis and DED.2-4 Patients with DED have been found to have elevated levels of MDA compared with patients without DED,5 and RASP levels have been associated with signs and symptoms of DED.6 The United States Food and Drug Administration officially named RASP as an objective sign of DED in June 2020.7
Reactive aldehyde species inhibition: a novel therapeutic mechanism of action
RASP molecules modulate inflammation at the top of the cascade and serve as gatekeepers of inflammation. In vivo sources of RASP include glycation, amino acid oxidation and lipid peroxidation, with non-enzymatic free radical mechanisms being the most prominent source.8 RASP induce alterations in enzymes and ion channels and produce pro-inflammatory substrates, including tumour necrosis factor alpha and cyclooxygenase-2. They induce cellular necrosis and apoptosis, DNA damage and amplification of the cascade by the production of new electrophilic aldehydes.9 While some RASP only exist for seconds, others exist long enough to modify proteins, membranes and DNA.10 RASP create DNA adducts, such as Michael addition of deoxyguanosine by 4–hydroxynonenal (4-HNE);10 Schiff base reactions with cysteine’s thiol group; and secondary amines of lysine, histidine and arginine.8,11
Cellular accumulation of 4-HNE modulates cell death and has been associated with diabetes, and liver and neurodegenerative diseases.10 Lipid–rich membranes, microsomes and mitochondria tend to have the highest accumulations of RASP. Elevated levels of RASP are associated with cardiovascular disease, diabetes,12,13 Behçet’s disease,14 allergy,15 uveitis16 and DED.4 Manipulation of the ALDH2 gene, which is responsible for aldehyde metabolism, has been investigated for pain management.17
RASP inhibitors are believed to block NF-kB translocation, scavenger receptor A binding and inflammasome activation, in turn blocking cytokine release. This blockage of inflammation reduces pro-inflammatory cytokine and histamine levels.1 It occurs earlier in the pathway prior to targets for non-steroidal anti-inflammatory drugs, steroids and immunomodulators. Reproxalap’s anti-inflammatory effect is being investigated for use in ocular conditions, including non-infectious anterior uveitis (NAU), allergic conjunctivitis and DED.
Clinical trials for anterior uveitis
In the initial study investigating the effect of reproxalap on anterior uveitis, Mandell et al. compared reproxalap with topical corticosteroids in patients with mild–to–moderate NAU.18 Forty-five patients were randomized to receive reproxalap 0.5% ophthalmic solution four times daily (QID) for 6 weeks; prednisolone 1% ophthalmic solution, initially QID and tapered over 6 weeks; or reproxalap solution 0.5% QID for 6 weeks, combined with prednisolone acetate 1% starting twice daily (BID) and tapered through week 4. Masked investigators graded standard signs and symptoms of anterior uveitis at weeks 1, 2, 4 and 8. The reproxalap group reached average treatment success time faster than the prednisolone and combination therapy group. Rescue therapy was required in 2/15 of the reproxalap monotherapy group, 4/16 of the combination group and 3/14 of the prednisolone monotherapy group. No statistically significant difference was found between the groups at week 8, and both reproxalap monotherapy and combination therapy were non–inferior to prednisolone alone. Intraocular pressure was elevated >10 mmHg in two patients (7%) who recieved prednisolone, while no average intraocular pressure increase was noted in the reproxalap monotherapy groups.
The SOLACE trial (ClinicalTrials.gov identifier: NCT03131154), a subsequent randomized, vehicle-controlled, double-blind clinical trial, was conducted to evaluate the safety and efficacy of reproxalap 0.5% on anterior chamber cell and flare in NAU compared with the drug vehicle.19 A total of 125 subjects were treated using either reproxalap 0.5% or vehicle for 4 weeks. An improvement in anterior chamber cell count was not achieved with statistical significance; however, the drug demonstrated improvement over the vehicle, and so the research focus shifted to the treatment of allergic conjunctivitis and DED.
Clinical trials for allergic conjunctivitis
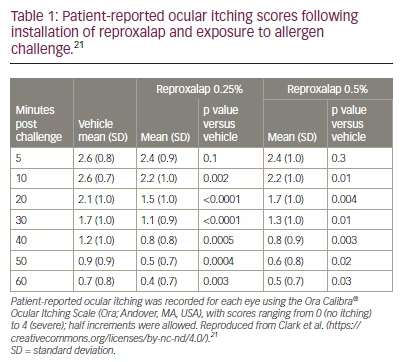
A phase II, randomized, double-blind, vehicle–controlled, crossover clinical trial (ClinicalTrials.gov identifier: NCT03709121) showed superiority of reproxalap versus the drug vehicle for the treatment of allergic conjunctivitis.20 The eligibility criteria of the trial included a positive test for ragweed pollen allergy and a history of chronic moderate–to–severe allergic conjunctivitis. The patients (n=70) were randomized (1:1:1) to one of three sequences: 0.25% reproxalap, 0.5% reproxalap and placebo (sequence A); 0.5% reproxalap, placebo and 0.25% reproxalap (sequence B); or placebo, 0.25% reproxalap and 0.5% reproxalap (sequence C). Patients attended three treatment visits, during which they received one drop of each test article in each eye approximately 1 minute before entering an allergen chamber, followed by another drop 90 minutes later. Patients were given forced visual tasks while ragweed pollen was continually aerosolized into the chamber for 3.5 hours. Patients ranked symptom scores at multiple time intervals for ocular itching and tearing, and masked investigators assessed conjunctival redness. The results showed that both concentrations of reproxalap improved itching, tearing and conjunctival redness. Reproxalap 0.5% caused a transient increase in symptom scores and redness, likely due to acute instillation discomfort. The effect of 0.5% was slightly but not significantly less than 0.25% (Table 1).21
Another randomized, double-masked, vehicle-controlled phase II trial of patients with ragweed-associated allergic conjunctivitis was performed over 28 days (ClinicalTrials.gov identifier: NCT03660878).22 Fifty-two patients received either 0.25% reproxalap, 0.5% reproxalap or the drug vehicle approximately QID. Although the study was limited by the overall low–pollen counts, reproxalap treatment groups showed improvement in ocular itching and ocular tearing scores on high-pollen days.
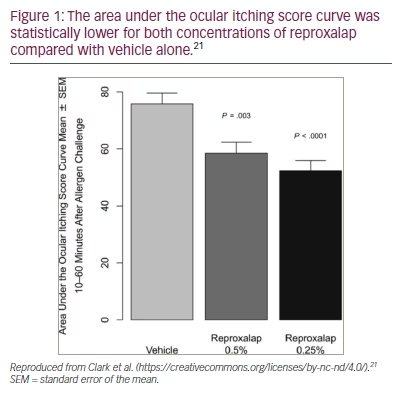
The ALLEVIATE trial (ClinicalTrials.gov identifier: NCT03494504), a phase III, double–masked, randomized, parallel-group study, evaluated the post-acute activity and clinical utility of reproxalap for treating ocular itching in seasonal allergic conjunctivitis.21 The primary endpoint was area under the post-acute ocular itching score curve from 10 to 60 minutes after allergen challenge. A total of 318 subjects were randomized to receive one drop of reproxalap 0.25%, 0.5% or placebo 10 minutes prior to exposure of the conjunctival allergen. Patients scored ocular itching at 10–minute intervals over 1 hour. In the study, the primary endpoint was achieved, with both concentrations of reproxalap improving symptoms of ocular itching at every time interval (Figure 1).
Clinical trials for dry eye disease
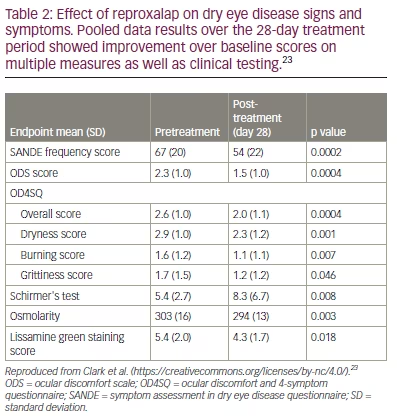
Reproxalap showed a decrease in tear RASP levels following 28 days of treatment in a phase IIa trial. Clark et al. conducted a randomized, double-masked, parallel-group trial to assess the safety and efficacy of reproxalap for DED (ClinicalTrials.gov identifier: NCT03162783).23 Fifty-one participants were randomized (1:1:1) to receive either reproxalap 0.1%, reproxalap 0.5% or reproxalap 0.5% lipid solution QID for 1 month. No vehicle–only group was included. Follow–up visits were scheduled at 1 week and 4 weeks to assess multiple subjective and objective DED measurements. All treatment arms showed significant improvement in scores such as lissamine green staining (p=0.002), Schirmer‘s test (p=0.008) and tear osmolarity (p=0.003). Additionally, the scores from the ocular discomfort and 4-symptom questionnaire, the ocular discomfort scale and the symptom assessment in DED questionnaire were all improved from baseline (Table 2).23 Specific RASP MDA levels in the tear film were lower post treatment, and lower MDA levels were associated with decreased conjunctival lissamine staining and decreased ocular surface osmolarity. Safety and tolerability were also established, with the main adverse event being transient instillation site pain. Ten patients in the 0.5% reproxalap group (10/34 patients) discontinued the study due to transient ocular irritation.
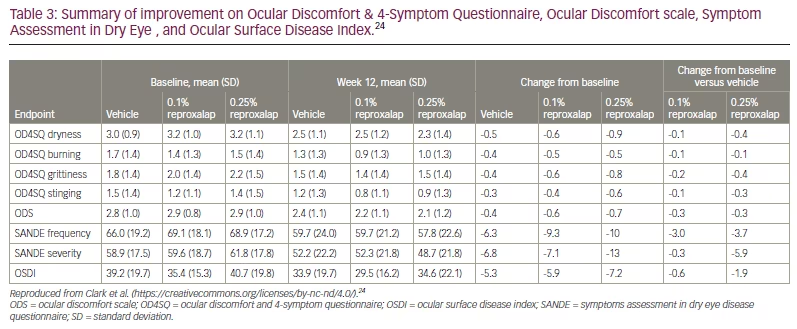
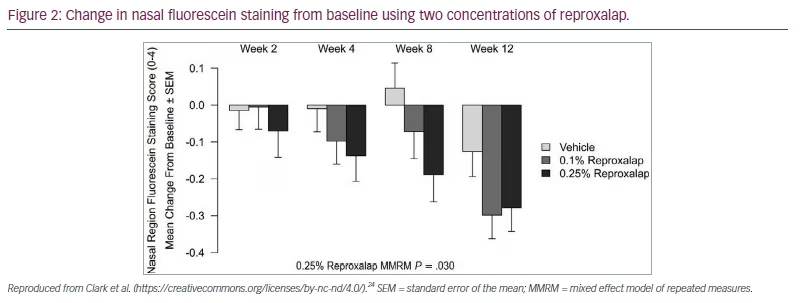
The randomized, double–masked, vehicle-controlled, multicentre phase IIb trial (ClinicalTrials.gov identifier: NCT03404115) investigated reproxalap compared with the drug vehicle in patients with moderate–to–severe DED.24 A total of 300 patients were randomized 1:1:1 to receive reproxalap 0.1%, reproxalap 0.25%, or the vehicle 1 drop QID for 12 weeks. At the two-week visit, patients in the treatment group with above–average baseline symptom scores had a statistically significant improvement over the control subjects for all symptom scale scores (Table 3).24 By week 12, all symptom scores were improved in the reproxalap 0.25% group. Nasal region fluorescein staining was the most improved objective sign (Figure 2).
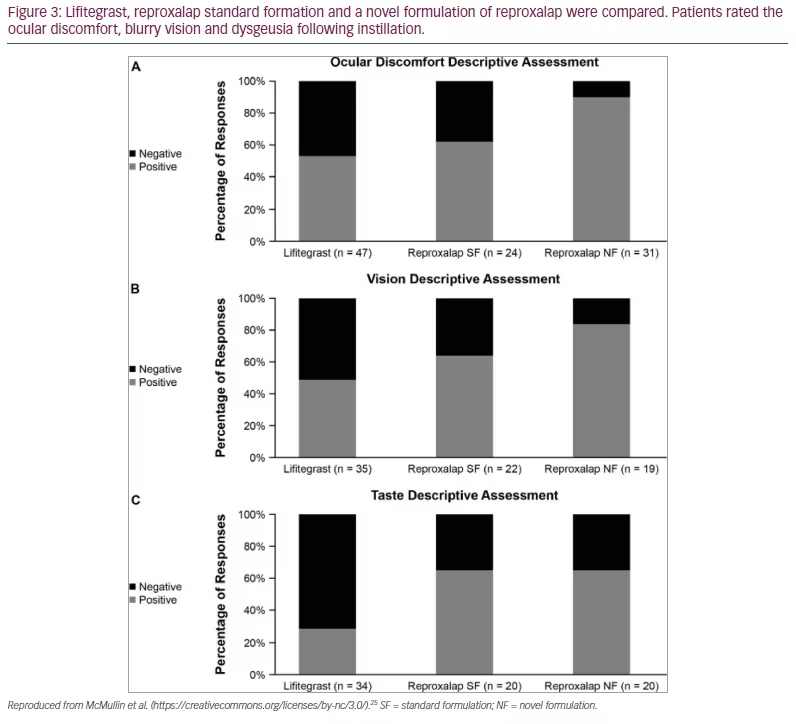
Reproxalap solution 0.25% was also compared with lifitegrast solution 5% in a study to assess its tolerability.25 Nineteen patients with DED were evaluated in a single-centre, double-masked, active-comparator, single-dose crossover trial, with 17 patients completing the trial. Patients were 18 years or older with a reported history of DED for <6 months. Ocular discomfort, dysgeusia and blurred vision were subjectively measured for 1 hour post drop instillation. These symptoms are commonly reported with lifitegrast ophthalmic solution 5%. Each patient underwent three visits. At each visit, one treatment was instilled to both eyes using an unmasked administrator: lifitegrast ophthalmic solution 5%, reproxalap 0.25% standard formulation (SF), or a novel formulation (NF) of 0.25% where the weight/volume of an excipient was modestly increased compared with the SF.
In this crossover trial, reproxalap caused patients less instillation discomfort and less dysgeusia than lifitegrast.25 Discomfort was defined as an ocular discomfort score of ≥3 at any time–point tested. Odds of such a response were higher in the lifitegrast group (44.9%) versus the reproxalap SF (10.5%; odds ratio [OR] 0.14; p=0.0006) and reproxalap NF groups (7.4%; OR 0.10; p=0.0003). Length of time of discomfort was also shorter for the reproxalap solution groups. Over all time points, the odds of negative dysgeusia responses (score ≥3 at any time-point tested) were higher in the lifitegrast group (10.2%) than in the reproxalap SF (2.0%; OR 0.18; p=0.0035) and reproxalap NF groups (1.0%; OR 0.09; p<0.0001). The average blurry vision rating (score ≥3) was significantly better for the reproxalap solutions (reproxalap SF: 0%, OR 0.25, p=0.0177; reproxalap NF groups: 0%, OR 0.21, p=0.0130) compared with lifitegrast (0.1%). Figure 3 illustrates the descriptive assessment for the three groups.25
The TRANQUILITY-2 trial (ClinicalTrials.gov identifier: NCT05062330), a phase III vehicle–controlled, randomized, double–masked trial, studied reproxalap 0.25% versus vehicle in 361 participants.26 The treatment group was administered one drop of reproxalap solution 0.25% seven times over 2 consecutive days, while the control group received one drop of the vehicle seven times over 2 consecutive days. Primary outcome measures included overall mean change in Schirmer’s test results and change of <10 mm in Schirmer’s test from baseline. Conjunctival redness was assessed using digital photography over 90 minutes in the DED chamber. Positive results have been reported by the manufacturer, Aldeyra Therapeutics, but not published to date.27–29
RENEW, a multicentre, randomized, triple-masked, vehicle–controlled, phase III clinical trial assessing the safety and efficacy of reproxalap in patients with DED has also been completed (ClinicalTrials.gov identifier: NCT03879863).30 Subjects were randomized to receive one of the following: reproxalap solution 0.25% administered QID for 12 weeks; vehicle ophthalmic solution administered QID for 12 weeks; reproxalap ophthalmic solution 0.25% administered QID for four weeks, followed by BID for 8 weeks; or vehicle ophthalmic solution administered QID for 4 weeks, followed by BID for 8 weeks. Primary outcome measures were subject–reported ocular dryness scores (0–100 visual analog scale) and fluorescein nasal region score, which was assessed using the Ora Calibra® scale (Ora; Andover, MA, USA). Secondary outcome measures were overall fluorescein staining and unanesthetized Schirmer’s testing. The results of the RENEW trial have not been published yet.
Conclusion
Ocular allergy and DED often have overlapping symptomatology, and reproxalap’s novel mode of action appears to provide a broad spectrum of relief for clinical signs and symptoms of both conditions. Non–steroidal formulations with less discomfort upon instillation would be appreciated by both clinicians and patients. On 29 November 2022, Aldeyra Therapeutics announced that a new drug application was submitted to the United States Food and Drug Administration for topical reproxalap, for the treatment of signs and symptoms of DED.31







