The subspecialty of the retina has been transformed by anti-vascular endothelial growth factor (anti-VEGF) medications over the last two decades.1 These include bevacizumab (Avastin®, Genetech, San Francisco, CA, USA), ranibizumab (Lucentis®, Genentech, San Francisco, CA, USA), aflibercept (Eylea®, Regeneron Pharmaceuticals, Tarrytown, NY, USA), brolicizumab (Beovu®, Novartis, Basel, Switzerland) and faricimab (Vabsymo™, Genentech, San Francisco, CA, USA), along with the ranibizumab port delivery system (Susvimo™, Genentech, San Francisco, CA, USA). These medications have demonstrated profound success in preserving and even improving vision in a variety of diseases, including neovascular age-related macular degeneration (NvAMD), diabetic macular oedema (DMO) and myopic choroidal neovascularization (mCNV).
The recent expiration of the ranibizumab patent in 2020, and the pending expiration of the aflibercept patent, has allowed for the introduction of biosimilars. Recently, the US Food and Drug Administration (FDA) approved a ranibizumab biosimilar, Byooviz™ (Samsung Bioepis, Incheon, South Korea), while many other biosimilars are in the pipeline.1 In addition to these medications being approved by agencies such as the European Medicines Agency (EMA), developing countries are also capitalizing on the availability of these medications, and spearheading a large number of biosimilar studies.2
The exact definition of a biosimilar is important to understand, especially to differentiate biosimilars from generic medications or a recently coined term ‘biobetters’.3 For the purposes of this discussion, the initial medication, such as ranibizumab, will be referenced as an ‘original’ or ‘innovator’ drug throughout this review. A biosimilar, in brief, is a non-exact replica of the original drug produced by a different company, and has similar biological characteristics to the original drug. Contrastingly, generic medications are exact copies of the active ingredients.3 Given the slight differences between biosimilars and their original molecule, biosimilars are prone to more heterogeneity in real-world practice as compared to their original counterparts.1 The term biobetters refers to a medication that targets the same protein as a pre-existing compound, but outperforms the original medication in side-effect profile, efficacy or longevity.3
Compared with innovator medications, which require two phase III trials to be approved, biosimilar medications only require one phase III trial that compares it to the original medication. The treatment indications can also be generalized beyond the disease studied in their clinical trials, which differs from the approval process for original medications. The FDA and EMA approve biosimilars based on the evidence that the biosimilar displays no clinical difference in its efficacy, safety and side-effect profile to the innovator drug, among other factors.4 Given the lesser requirements for approval, the price and time investment for developing biosimilars are less than for innovator drugs.1 The large financial burden that comes with current anti-VEGF therapies will likely demand a more fiscally sustainable option; biosimilars have an expected high implementation rate, at least in developing countries, though usage in the United States remains to be seen.5
While biosimilars have only recently entered the ophthalmology scene, biosimilars have successfully been integrated into other medical specialties, such as oncology and dermatology, with the first biosimilar approved by the FDA in 2015.6 Multiple studies have investigated the use of biosimilars in a variety of retinal conditions, including NvAMD, DMO and mCNV, which this review will cover.7
Neovascular age-related macular degeneration, diabetic macular oedema and myopic choroidal neovascularization treatments
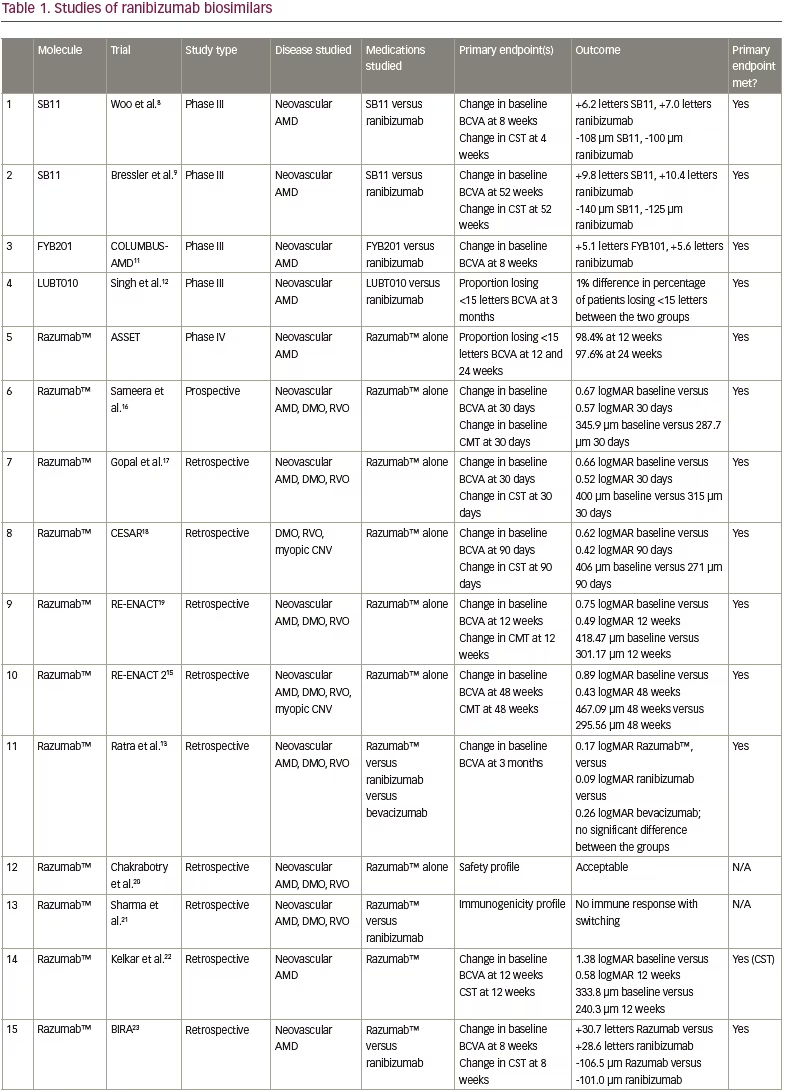
The standard of care for NvAMD, DMO and mCNV consists of anti-VEGF therapy. The drawbacks to this therapy are that the burden of treatment can be overwhelming and can carry intense financial ramifications. The high cost and a lack of visual improvement are the leading causes of anti-VEGF therapy discontinuation.1 Investigations into biosimilar alternatives to original drugs may provide a method to alleviate the financial burden. Multiple studies have evaluated, or are evaluating, ranibizumab biosimilars, with most studies focusing on Razumab™ (Intas Pharmaceuticals Ltd, Gujarat, India), an Indian biosimiliar. The results of these studies can be found in Table 1 and are discussed below.
SB11/Ranibizumab-nuna
SB11, also known as ranibizumab-nuna or Byooviz™, is approved by the FDA and EMA for NvAMD, retinal vein occlusion (RVO) and mCNV. It was assessed in a quadruple-masked, multicentre trial with 705 patients aged ≥50 years with treatment-naive, active, subfoveal CNV in AMD.8 The primary endpoint was change from baseline in best corrected visual acuity (BCVA) at 8 weeks, with SB11-treated patients gaining 6.2 letters and ranibizumab-treated patients gaining 7.0 letters (90% confidence interval [CI], -1.8 to 0.2). Additionally, change in central subfield thickness (CST) at 4 weeks was assessed (-108 μm for SB11 versus -100 μm for ranibizumab; 95% CI, -19 to 3 μm). Adverse events were similar between the two groups.8
Recently, the safety and side-effect profile of SB11 was evaluated in a phase III, multicentre, double-masked, 52-week study in 75 centres across nine countries in patients with NvAMD.9 BCVA gains were similar between the groups, with 9.8 letters gained in the SB11 group versus 10.4 letters in the ranibizumab group; while CST similarly decreased between the groups (140 μm in the SB11 group, 125 μm in the ranibizumab group). The extension of this trial to 52 weeks demonstrated no clinically consequential differences in the safety and efficacy profiles. No new safety concerns arose with the extension to 52 weeks, although a larger study is necessary to truly evaluate the side-effect profile, including intraocular inflammation.9
A recent study evaluating the analytical similarity between SB11 and the US and European Union versions of ranibizumab showed that the amino acid sequence was identical between the biosimilar and the innovator drug, with other parameters being very similar.10
FYB201
FYB201 (Formycon, Munich, Germany) was studied in the COLUMBUS-AMD trial for patients with NvAMD.11 Inclusion criteria required participants to be over the age of 50 years with an active, subfoveal CNV who were treatment naive. This quadruple-blinded multicentre trial randomized patients in a 1:1 ratio to either FYB201 or ranibizumab, with the primary endpoint being the change from baseline BCVA at Week 8, with similar outcomes between both groups. The mean BCVA improved in both the FYB201 group (+5.1 letters) and the ranibizumab group (+5.6 letters; 90% CI, -1.6 to 0.9). In terms of secondary outcomes, CNV leakage was also measured with similar outcomes; by Week 48, 56.4% of the FYB201 group and 58.7% of the ranibizumab group had leakage. There was no difference in the ocular or systemic adverse events between the groups.11
LUBT010
LUBT010 is a ranibizumab biosimilar developed by Lupin, Mumbai, India. It was tested against ranibizumab in a phase III, prospective, multicentre, randomized trial of 202 patients with NvAMD, with the primary endpoint measuring the percentage of patients losing less than 15 letters at 3 months. The groups had similar rates of losing 15 letters or less, with a 1% difference between the groups.12
Razumab™
Razumab™ is a humanized, recombinant monoclonal antibody produced through Escherichia coli.13 It has been studied extensively in India in NvAMD, DMO and mCNV, along with other considerations, in both prospective and retrospective studies. According to one report, the cost of Razumab™ is US$175, whereas ranibizumab costs US$322.13
ASSET, a phase IV, prospective post-marketing study enrolled 126 Razumab™-treated patients aged >50 years with NvAMD.14 The primary endpoints included the proportion of patients losing <15 letters in BCVA, and changes in central retinal thickness measured at Week 12 and 24; both were significantly improved at Week 12 and 24. Additionally, quality-of-life metrics significantly improved.15
Sameera et al. also assessed Razumab™ in a prospective study of patients with NvAMD, DMO or RVO.16 The study included 123 eyes of 95 patients, with the primary endpoints including change from baseline in BCVA on Day 30, and CST, with both showing significant improvement (BCVA 0.67 logarithm of the minimum angle of resolution [logMAR] versus 0.57 logMAR at baseline and 30 days, respectively; CST 345.9 μm versus 287.7 μm, respectively). Significant improvements were also seen in the subgroup analysis of NvAMD, DMO and RVO when analysed separately.16
Gopal et al. assessed Razumab™ in a retrospective study, showing a significant improvement in BCVA and CST at 30 days after a single dose in 309 eyes of 297 patients.17 A single-centre, retrospective review of 141 patients (153 eyes) with DMO, RVO or mCNV (CESAR) showed that Razumab™ improved BCVA and central macular thickness at 3 months.18 The RE-ENACT and RE-ENACT 2 studies retrospectively evaluated patients with NvAMD, DMO or RVO (with RE-ENACT 2 also including patients with mCNV).19,15 They assessed change from baseline in BCVA and CST at 12 weeks (RE-ENACT, with improvement noted from 0.75 logMAR to 0.49 logMAR) and 48 weeks (RE-ENACT 2, with improvement noted from 0.89 logMAR to 0.43 logMAR). Approval for the drug in India was based on the RE-ENACT data published in 2018.19
Other studies have focused on comparing ranibizumab biosimilars to original medications to assess their real-world outcomes and safety profile. A retrospective case series compared Razumab™, ranibizumab and bevacizumab in 202 patients with NvAMD, DMO or RVO.13 Primary outcomes included improvement in BCVA at 3 months, with all three groups showing statistically significant improvement, and with no significant difference noted among the three agents.13 The largest real-world outcomes study – a multicentre retrospective review of patients with NvAMD, DMO or RVO (6,404 eyes) – focused on the adverse event profile of Razumab™, with a total of 9,406 injections.20 Of these, 1,978 injections were associated with adverse events, with the serious ocular adverse effects including retinal pigment epithelial tears (31 cases; 0.33%), vitritis (2 cases; 0.02%) and endophthalmitis (1 case; 0.01%). Overall, adverse events were associated with 21.03% injections; however, most of these (97.12%) were non-serious. The authors concluded that Razumab™ therapy has an acceptable ocular and systemic safety profile in the real-world setting.20 The immunogenicity profile of switching patients from ranibizumab to Razumab™ was also assessed in 30 patients, with no concerns noted.21
Another study assessed the real-world outcomes of 12 weeks of Razumab™ treatment in NvAMD, with main outcomes including change in BCVA, retinal fluid, CST and pigment epithelial detachment.22 At 12 weeks, 90% of patients had maintained or improved BCVA, and resolution of sub- and intraretinal fluid occurred in 45% and 25% of eyes, respectively.22 The BIRA study evaluated the real-world outcomes of patients treated with Razumab™ for at least 8 weeks, and saw that there were no significant changes in BCVA and CST compared with patients treated with ranibizumab, and no significant adverse events were reported.23
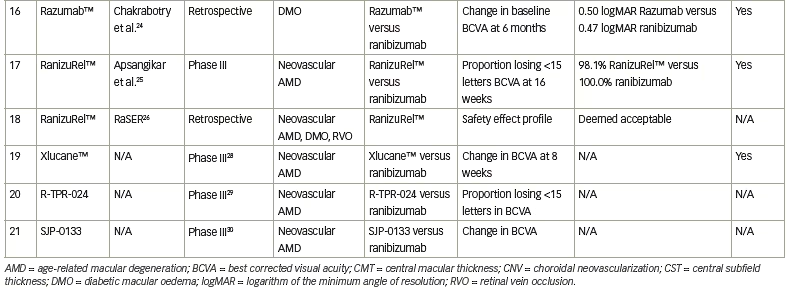
Finally, the real-world outcomes of Razumab™ compared with ranibizumab in DMO were retrospectively evaluated in 69 eyes (Razumab™) and 264 eyes (ranibizumab), with the primary outcome being change in BCVA at 6 months.24 Both groups showed significant improvement from baseline in BCVA, and no significant difference was shown between groups. CST also improved in both groups, without significant differences between them.24
RanizuRel™
Another Indian-produced ranibizumab biosimilar, RanizuRel™ (Reliance Life Sciences, Mumbai, India) displayed non-inferiority to ranibizumab for clinical profile, safety and immunogenicity.25 This was evaluated in a prospective, multicentre, randomized controlled trial that included patients ≥50 years with NvAMD. Overall, 160 patients were randomized 2:1 to biosimilar (107 patients) or ranibizumab (53 patients) and received treatment every 4 weeks for 24 weeks. The primary endpoint was the percentage of patients who lost ≤15 letters at Week 16. By Week 16, 98.1% of patients in the biosimilar arm and 100.0% of those in the ranibizumab arm had lost ≤15 letters; similar trends were noted at 24 weeks. None of these outcomes were statistically significant between groups.25 A real-world study also assessed the safety profile of RanizuRel™ in patients with RVO, DMO or NvAMD, without any safety concerns noted.26
Xlucane™
Xlucane™ is a ranibizumab biosimilar developed by Xbrane Biopharma, Solna, Sweden. A phase III, double-masked, multicentre parallel-group trial (XPLORE; ClinicalTrials.gov identifier: NCT03805100) 1:1 randomized approximately 580 patients with NvAMD to Xlucane™ or ranibizumab.27 The primary endpoint was change from baseline in BCVA at 8 weeks. The preliminary results at 6 months announced that the primary endpoints have been met.28
R-TPR-024
A phase III, multicentre, double-masked, prospective randomized trial (Clinical Trials Registry India identifier: CTRI/2018/05/014065) included 159 patients with NvAMD who were treated with either the ranibizumab biosimilar R-TPR-024 (Reliance Life Sciences, Mumbai, India) or ranibizumab.29 The results are not yet available.
SJP-0133
A phase III, randomized, single-blind trial of the ranibizumab biosimilar SJP-0133 (Senju Pharmaceutical, Osaka, Japan) is on-going (UMIN Clinical Trials Registry identifier: UMIN000030010).30 This trial of approximately 338 patients is comparing SJP-0133 to ranibizumab administered in a pro re nata fashion, with the primary outcome being change in BCVA.30 Results have not yet been released.
Conclusions
Real-world data are still lacking, as biosimilars have only been introduced recently, especially in the US population. Further, randomized clinical trial evidence is sparse.31 As biosimilar medications are not exact replicas of their innovator drug counterparts, they can have heterogeneity in their clinical profile, which raises concerns particularly for their side-effect portfolio. While implementing biosimilars in the retina realm remains to be seen, their reduced cost compared with innovator drugs, coupled with their promising results in various trials, adds to their viability.32
Additionally, the use of biosimilars also depends on physician comfort surrounding the drug, and the pharmacological competitors available, which can, in turn, impact the cost of biosimilars. In countries where the off-label use of compounded bevacizumab may be scarce, a biosimilar may be preferable. In a recently administered Bio-USER survey by Sharma et al., retinal physicians in the USA preferred to use bevacizumab off-label more than European retina specialists did, and preferred having real-world data before committing to using biosimilars.33 As the use of biosimilars increases, the real-world outcomes of the drugs, as well as their efficacy and adversity profiles, can be better understood and may influence their use, particularly in the USA.







