Neovascular age-related macular degeneration (nAMD) is characterized by the growth of abnormal blood vessels extending through Bruchs membrane in the macular region.1 This macular neovascularization (MNV) can be classified based on localization as either type 1, type 2, type 3 or mixed lesions.2 In type 1 MNV (also known as occult lesions), the neovascularization arises from the choroid to breech Bruch’s membrane and localize below the retinal pigment epithelium (RPE). In type 2 MNV, the neovascular lesion is located above the RPE in the subretinal space, whereas in type 3 MNV (also known as retinal angiomatous proliferation), the lesion extends from the middle retina toward the RPE. When any type of MNV activity produces exudation, haemorrhage or fibrosis, this can produce outer retinal and photoreceptor damage, resulting in permanent central vision loss. Multiple pathways have been identified in the pathogenesis of retinal vascular diseases. This article reviews an emerging treatment antibody targeting two important proteins implicated in the pathogenesis of nAMD, and describes how targeting a novel pathway aids in the development of treatments for this potentially disabling disease.
Targeting the pathways of nAMD pathogenesis
Vascular endothelial growth factor pathway
Preclinical research identified vascular endothelial growth factor (VEGF) as a key regulator in the process of retinal angiogenesis. VEGF is a vascular permeability factor, or signalling protein, in the platelet-derived growth factor family, with four subtypes, VEGF-A, -B, -C, -D, which are received by three transmembrane tyrosine kinase receptors, VEGFR-1, -2, -3. Each VEGF subtype can lead to a slightly different biological pathway, with the primary clinical research focussing on VEGF-A and -B, the subtypes most implicated in the growth and maintenance of new vasculature. VEGF-A binding to its receptor, VEGFR-2, is directly involved in angiogenesis. Inhibition of VEGF-A by anti-VEGF agents resulted in reduced leakage from choroidal neovascularization (CNV) in the primate model of laser-induced CNV, supporting VEGF-A as the primary target in the treatment of nAMD.3,4
The first intravitreal anti-VEGF agent approved by the US Food and Drug Administration (FDA) in December 2004 for the treatment of nAMD was pegaptanib (Eyetech, Long Island, NY, USA), a 50 kDa selective inhibitor of VEGF-A. It demonstrated efficacy in reducing progressive loss of vision in eyes with nAMD. Bevacizumab (Genentech/Roche, San Francisco, CA, USA) was approved by the FDA in Febuary 2004 as an intravenous systemic therapy for metastatic colon cancer. It is a monoclonal antibody with two VEGF-A binding sites. It was initially hypothesized that bevacizumab would not be able to diffuse into or under the retina owing to its larger size of 148 kDa. However, clinical studies demonstrated that intravitreal injection of 1.25 mg of bevacizumab, dosed every 4 weeks, was an effective treatment for eyes with nAMD, leading to improvement in visual acuity, retinal thickness and fluorescein angiographic leakage.5 However, bevacizumab is not currently FDA approved for intravitreal injection, although it is commonly used off-label in the USA to treat nAMD.
Ranibizumab (Genentech/Roche) is a recombinant monoclonal antibody fragment, with a smaller molecular weight of 48 kDa, targeting VEGF-A. It was designed to have a 100-times greater binding affinity for VEGF-A than bevacizumab with only a single binding site, and a smaller molecular weight to hypothetically potentiate retinal tissue penetration. Ranibizumab was FDA approved in 2006 for the treatment of nAMD. Following this, aflibercept (Regeneron, Tarrytown, NY, USA), a 115 kDa humanized, recombinant protein made from portions of human VEGFR, was FDA approved in 2011 for the treatment of nAMD.6 By combining the first binding domain of VEGFR-1 and the third binding domain of VEGFR-2, aflibercept binds these VEGF isoforms with greater affinity than natural VEGFR-1 or -2, while also binding placental growth factor, another protein implicated in angiogenesis. The most recent intravitreal molecule for nAMD, approved by the FDA in 2019, is brolucizumab (Novartis, East Hanover, NJ, USA). Brolucizumab is a single-chain antibody fragment targeting VEGF-A with a small molecular weight of 26 kDa, allowing high molar concentration and potentially effective tissue penetration.7
Angiopoietin pathway
Although multiple intravitreal therapeutics are available for nAMD, the primary target for these approved agents remains VEGF-A. This limits the breadth of treatment options for patients who demonstrate a suboptimal response to the anti-VEGF agents, which may be as high as 25% of eyes with nAMD.8 The unmet need for new treatment options led to a focus on alternative mechanisms such as the angiopoietin pathway, which plays a role in angiogenesis and is implicated in vascular disease. The angiopoietin–tyrosine-protein kinase system is a protein–receptor system that plays a complementary role in regulating vasculature alongside VEGF. Angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2) are two distinct vascular growth factors that bind to the Tie-2 transmembrane receptor. Each molecule leads to different reactions when bound. Ang-1 is a full agonist of Tie-2 and results in phosphorylation of the receptor, thus inhibiting vascular permeability and preserving vascular stability.9 By contrast, Ang-2 acts as either a partial agonist or antagonist of Ang-1 that inhibits phosphorylation of the Tie-2 transmembrane receptor, thereby deactivating the entire pathway and disrupting vascular stability.10 Although both Ang-1 and Ang-2 bind to the same receptor, each agent produces opposing vascular effects.
In pathological conditions such as hyperglycaemia, hypoxia or oxidative stress, Ang-2 is known to be markedly upregulated.11 Ang-2 effectively competes with Ang-1 for the Tie-2 receptor, thus inhibiting Ang-1-induced Tie-2 activation. In conjunction with the upregulation of VEGF in similar disease states, co-expression of these two permeability factors (Ang-2 and VEGF) has been reported to accelerate neovascularization in the developing retina and ischaemic retina models.12 Recombinant Ang-1, which is analogous to natural Ang-1 with regard to its vascular stability function, was used as a possible injectable treatment for abnormal angiogenesis in mouse models.13 Findings included decreases in capillary vasoregression, vascular permeability and retinal hypoxia, supporting inhibition of Ang-2 function by its balancing factor.
Development of faricimab
With both VEGF-A and Ang-2 as primary targets, Genentech developed faricimab (formerly RG7716), the first novel bispecific antibody designed for intravitreal use that simultaneously binds and neutralizes VEGF and Ang-2.14 By targeting both molecules, faricimab has the potential to reduce leakage and inflammation, while stabilizing vasculature. In addition to efficacy, the dual inhibition by faricimab along two target pathways hopes to improve durability and sustainability of treatment, thereby reducing the treatment burden for patients who require frequent intravitreal injections to manage nAMD.
Preclinical and phase I studies
Preclinical studies revealed that spontaneous CNV treated with faricimab resulted in reduced vessel lesions and neurone loss, and that inhibition of Ang-2 reduced VEGF-induced endothelial barrier breakdown.15–17 A successful phase I clinical trial in patients with nAMD confirmed that faricimab had a good safety and tolerability profile, and even showed improvements in best corrected visual acuity (BCVA) and anatomical parameters.18 With these successful results, faricimab was moved into phase II trials.
Phase II studies
Two phase II studies, AVENUE and STAIRWAY, have evaluated the safety, dosing and preliminary efficacy of faricimab for treatment of nAMD (Table 1). The AVENUE trial (ClinicalTrials.gov Identifier: NCT02484690) evaluated 273 treatment-naïve eyes with MNV secondary to nAMD. Eyes were randomized into one of five cohorts: 0.5 mg ranibizumab control every 4 weeks; 1.5 mg faricimab every 4 weeks; 6.0 mg faricimab every 4 weeks; 6.0 mg faricimab every 8 weeks after four monthly loading doses; and a 0.5 mg ranibizumab group receiving three monthly loading doses then switched to 6.0 mg faricimab every 4 weeks.19 The primary outcome measure evaluated mean change in BCVA, measured by Early Treatment Diabetic Retinopathy Study (ETDRS) letters at week 36, with secondary outcomes including central subfield thickness (CST) changes, anatomic parameters measured by optical coherence tomography and other vision criteria. All cohorts demonstrated visual acuity and CST improvements, but faricimab was not superior to ranibizumab.
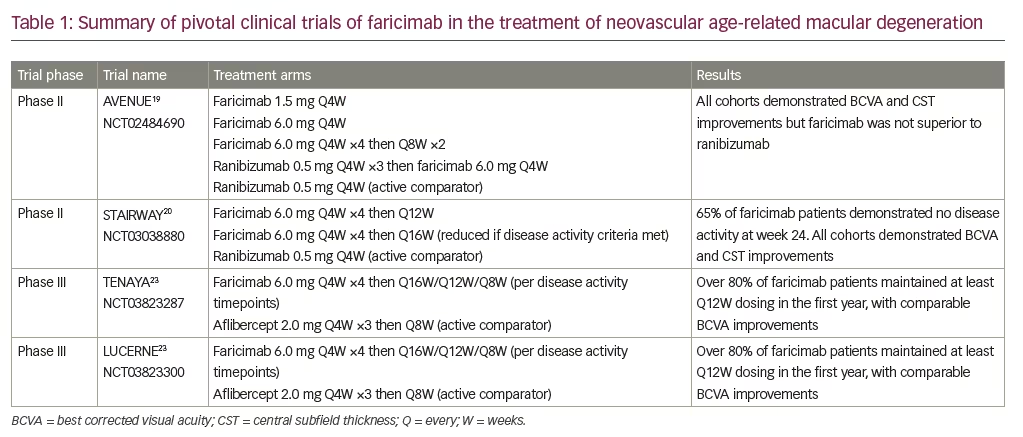
In the 52-week STAIRWAY study (ClinicalTrials.gov Identifier: NCT03038880), 76 treatment-naïve eyes were evaluated in extended-dosing cohorts for faricimab. Patients were enrolled in one of three cohorts: 6.0 mg faricimab every 16 weeks after four monthly loading doses; 6.0 mg faricimab every 12 weeks after four monthly loading doses; and 0.5 mg ranibizumab every 4 weeks.20 Approximately 65% of eyes in the faricimab treatment group demonstrated no disease activity at week 24, which was 12 weeks after the last loading dose. At the final week 52 study visit, the mean ETDRS letter improvement was +11.4, +10.1 and +9.6 in the 16-week faricimab group, 12-week faricimab group and monthly ranibizumab cohort, respectively. Comparable improvements in CST were noted for all three treatment groups, with no notable safety signals. The safety, potential durability and efficacy signals from extended dosing of faricimab led to the initiation of phase III clinical faricimab trials.
Phase III studies
Faricimab is currently being evaluated in two phase III clinical trials in relation to nAMD, TENAYA (ClinicalTrials.gov Identifier: NCT03823287) and LUCERNE (ClinicalTrials.gov Identifier: NCT03823300). These two studies evaluated over 1200 treatment-naïve patients with nAMD (Table 1).21 Both trials were initiated in 2019, and were multicentre, randomized, double-masked studies aimed at evaluating efficacy, durability and safety of intravitreal injection of faricimab 6.0 mg compared with aflibercept 2.0 mg for treatment of patients with nAMD. Eyes in the faricimab groups received four monthly loading doses and were then placed in one of three treatment interval groups (faricimab every 8 weeks, 12 weeks or 16 weeks) based on disease activity assessment at weeks 20 and 24. Eyes in the aflibercept group were treated with three monthly loading doses then every 8 weeks as per the FDA-approved label regimen. Patients were seen every 4 weeks throughout the entire study and sham injections were performed at every non-treatment visit to maintain masking between the cohorts. The primary endpoint was non-inferiority of faricimab compared with aflibercept, as evaluated by change in ETDRS letter score from baseline averaged over three time points at weeks 40, 44 and 48 to allow for the varied treatment intervals. Both studies have completed patient enrolment and the data for primary analysis were released in Q1 of 2021.
Both TENAYA and LUCERNE trials met their primary endpoints, as faricimab demonstrated non-inferiority to aflibercept in ETDRS letter improvement in vision.22,23 Mean ETDRS letter gains across both trials in the faricimab arms were +5.8 and +6.6 letters, respectively, compared with +5.1 and +6.6 letters in the aflibercept arms. The durability of faricimab was also evaluated by calculating the proportion of patients in the faricimab arms who maintained extended-dosing intervals of every 16 weeks or 12 weeks. Overall, 45.7% of patients in TENAYA and 44.9% in LUCERNE remained on the 16-week dosing interval. Additionally, 34.0% and 32.9% of patients in TENAYA and LUCERNE, respectively, were extended to 12-week dosing. When combined, nearly 80% of all patients in the faricimab arms of both trials required treatments only every 12 weeks or longer during the first year, with faricimab up to the 16-week dosing interval demonstrating similar CST reduction to aflibercept at the 8-week dosing frequency.
Conclusion
These recent promising data relating to durability and efficacy may pave the way for faricimab to join the armamentarium of treatment for nAMD. The novel bispecific therapeutic design of faricimab enables a single molecule to effectively neutralize both VEGF and Ang-2 as supported by preclinical in vitro and animal models. This may provide an additional target pathway for patients who have not responded favourably to anti-VEGF monotherapeutic agents. Faricimab has also demonstrated superior durability compared with approved agents in current clinical trials, with 80% of patients having their dosing interval extended to 12–16 weeks after loading doses. These data support the potential of faricimab to reduce the treatment burden for naïve as well as previously treated patients with nAMD. Further exploration of the phase III data will add to the data supporting the potential of faricimab as a new therapy for nAMD.







