Optical coherence tomography (OCT) has proved to be a valuable approach to imaging ocular vasculature and is becoming a critical method in precisely diagnosing the type and identifying the location of retinal disease.1–7 Fluorescein angiography (FA), used to examine the retinal microvasculature, to date has produced valuable diagnostic information and insights into the pathophysiology of retinal disease. FA continues to be an important diagnostic method in retinal disease but its use necessitates an intravenous injection of dye for each analysis. The advantage of OCT angiography is that it is a rapid, higher-contrast and less-invasive technique, which helps to diagnose and monitor retinal disease and treatment. There are now more than three approaches to OCT angiography: this article reports the application of the new AngioPlex OCT angiography technology on CIRRUS HD-OCT in specific pathologies. Specific cases of its application were discussed at a symposium convened by ZEISS in Nice, France during the September 2015 EURETINA Congress.
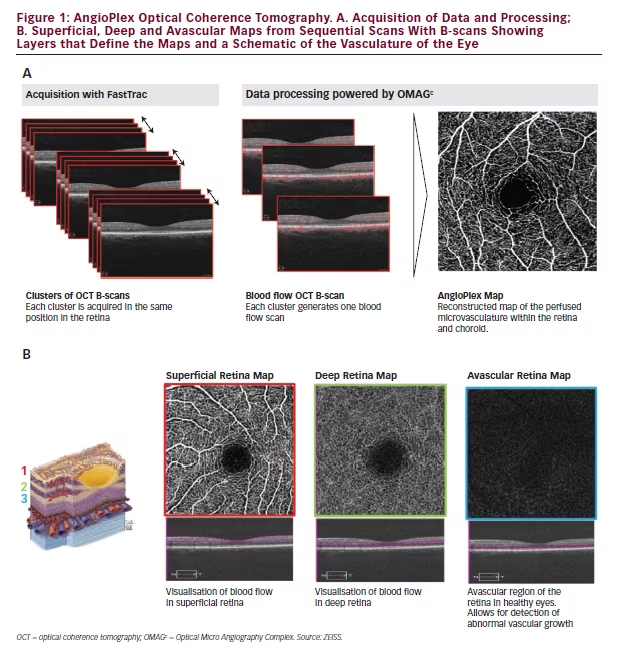
AngioPlex Optical Coherence Tomography Angiography – An OverviewAngioPlexTM OCT angiography is a new technology that runs on the ZEISS CIRRUS HD-OCT instruments, and can be enabled on existing instruments with new software and minor hardware upgrades. This approach uses the entire OCT signal, and utilises FastTracTM and Optical Micro Angiography Complex [OMAGC] algorithms to visualise microvasculature. AngioPlex uses both amplitude and phase aspects of the OCT signal, which is different from other systems that only use one. A scanning laser diode scans each retinal position up to four times and eye motion during the scans is corrected with live-tracking using FastTrac technology (see Figure 1). Although the OCT angiography field of view is limited to 3 mm by 3 mm or 6 mm by 6 mm, the scan can be positioned over an area of neovascularisation. FastTrac allows this area to be scanned on subsequent visits to monitor changes with progression of disease, or
changes indicating response to treatment. Monitoring over multiple visits is seldom performed with FA due to the invasive nature of the procedure.
OMAGC measures the differences between signals at successive locations and represents this as vectors, which are used to create a picture of changes in the microscopic structures encountered. The image produced is based entirely on contrast in the OCT signal created by flow, not on the presence of a dye as is the case with FA or indocyanine green angiography (ICGA). The process is highly sensitive, but is also robust and reliable, being able to correct for blinks and eye motion during the scanning using real-time tracking. AngioPlex uses the repeated scans as well as segmentation algorithms to build up a series of ‘slabs’ or ‘maps’ of the superficial retina, the deep retina, avascular retina choriocapillaris and choroid (see Figure 1). These maps are highly accurate, detailed 2D representations of 3D structures of the entire retinal microvasculature, including blood vessels that were previously difficult or impossible to observe. Image selection and export to reports and other media is simple and the captured pictures can be readily shared between ophthalmologists and used for teaching purposes.
AngioPlex OCT angiography provides an improved patient experience. Image acquisition is rapid and the scanning procedure is completed within seconds. AngioPlex OCT angiography is a non-invasive process that also increases patient workflow in ophthalmic practices while increasing the quality and depth of imaging provided. AngioPlex OCT angiography is able to separately visualise vessels of the superficial retina, the deep
retinal layer, the choriocapillaris and the other large choroidal vessels. It can be used to detect choroidal neovascularisations (CNVs) in conditions such as age-related macular degeneration (AMD), or retinal abnormalities in macular telangiectasia.8–11 It can also be used to examine ischaemia in DR, occlusions and abnormalities in central and branch vein retinal occlusion and in abnormalities of optic nerve vasculature.4,12–20
Limitations of Fluorescein Angiography
FA also involves intravenous injection of a contrast dye. Within the retinal vasculature, it can enable the visualisation of both the superior and deeper layers where evidence of disease may be located but cannot differentiate between them.21,22 FA does allow the visualisation of rapid capillary leakage but this can prevent observation of capillary nets.
Angiographic Imaging of Retinal Vasculature in Specific Ophthalmic Conditions – Case Examples
Diabetic Retinopathy
In DR, capillary closures and microaneurysms occur in the retinal vasculature. This exudate, AngioPlex OCT can be successfully used to observe the microvasculature in cases of DR and allow more informed treatment decisions and monitor patient response and progress. The visualisation of the superficial and deep capillary nets and their alterations obtained using AngioPlex is clearly much better than with FA.
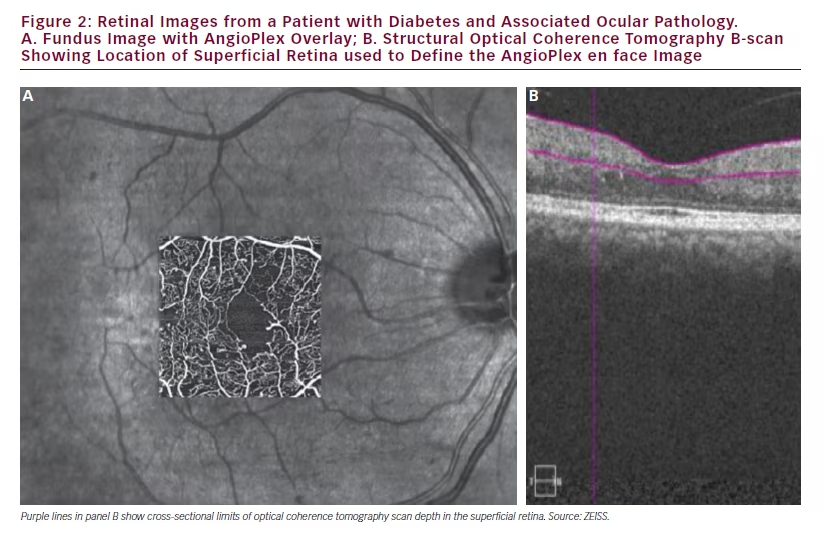
In cases of diabetes abnormalities on photographic images of the fundus and on FA images can be detected but, with these alone, it is difficult to assess perfusion of the fovea. Much greater detail and more accurate assessments of disease can be made using AngioPlex OCT angiography as shown in Figure 2 in which the AngioPlex OCT angiography foveal image of a patient with diabetes is overlaid on the FA image for comparison purposes. The increased detail and visualisation of individual capillaries and occlusions is clear. These pictures provide greatly increased information but further understanding and interpretation of the processes they reveal is required.
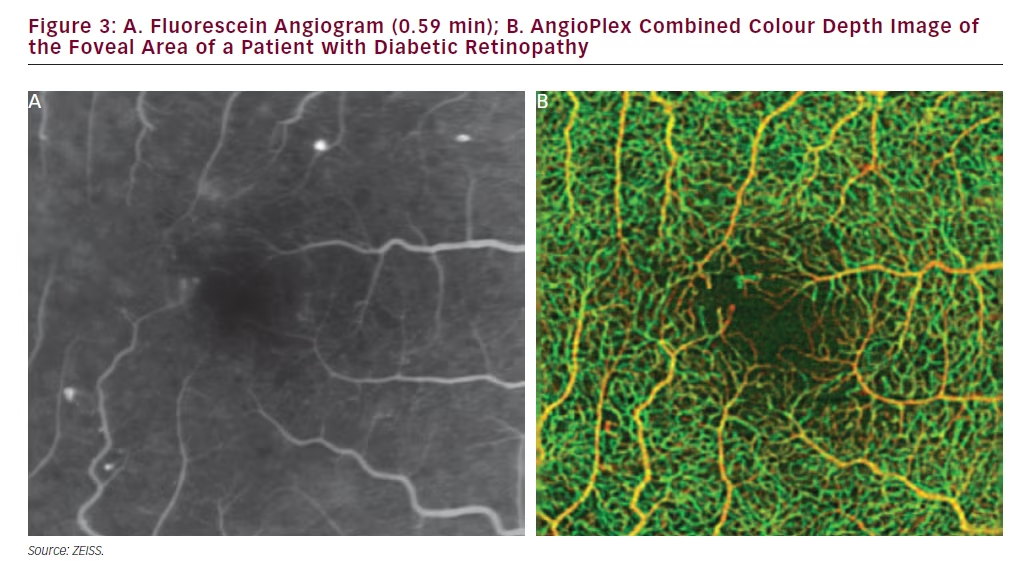
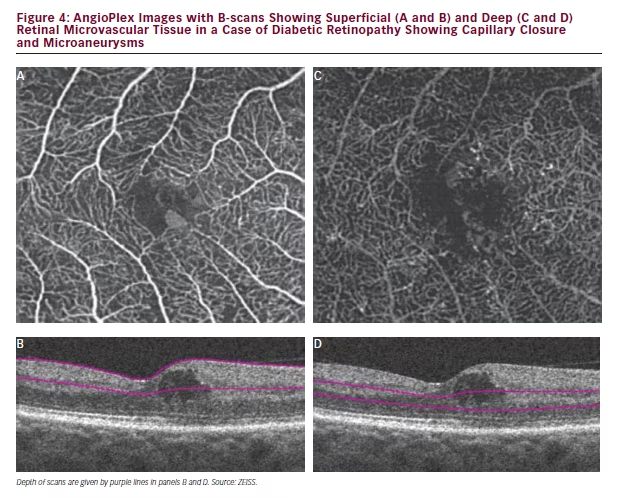
In the example of a case of DR represented in Figure 3 (Early Treatment Diabetic Retinopathy Study [ETDRS] level 35), the limited information provided by FA is apparent when compared with the OCT angiography. In the FA some capillary closure or microaneurysms can be observed but these are difficult to identify precisely. It is clear that much detail, especially of the deep retina, is missing. The colour map produced by AngioPlex OCT angiography, however, shows substantially more detail that extends to deeper layers enabling better visualisation of disease. In this image the orange structures are closer to the surface and the green structures are at deeper positions giving a three-dimensional perspective and an understanding of disease location. In this case, AngioPlex OCT enables visualisation that is entirely new; vasculature that was previously invisible can now be examined. In a further case of DR (ETDRS level 43, Figure 4), it can be seen in scans of the foveal avascular zone that the deep retinal area appear denser than normal and there is clear evidence of capillary closure and microaneurysms in both the superficial and deep retinal zones. AngioPlex OCT angiography images provide a wealth of information but interpretation of the specific structures revealed has not been fully established; definitions of the observed pathological manifestations are likely to improve as experience in real-world patient care increases.
Vein Occlusions
In cases of vein occlusion examined using FA, changes to the foveal microvasculature can be observed but it is difficult to determine the extent of the disease and make a quantitative assessment at different
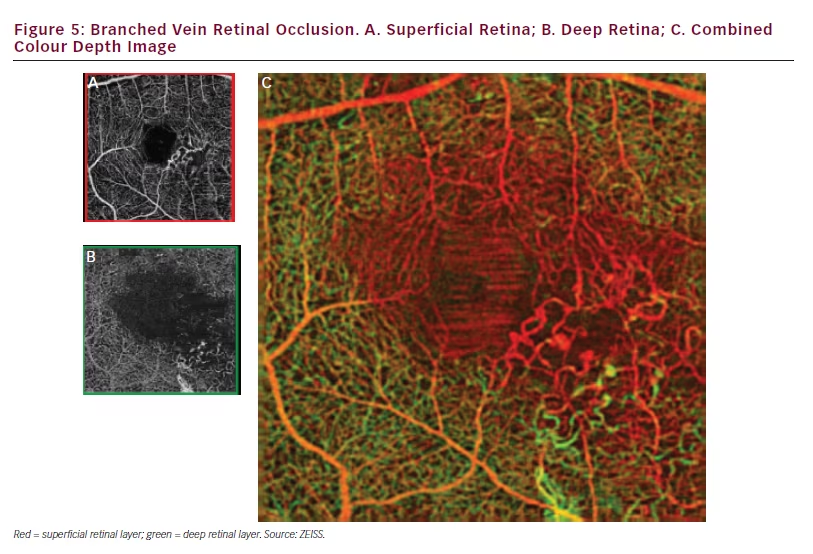
retinal depths. With AngioPlex OCT angiography images, the degree of detail provided and the ability to view deep retinal areas makes such assessment easier. A case of branched vein retinal occlusion, when viewed using AngioPlex OCT angiography, shows 30–40 % of blood vessels in the circle around the fovea are damaged in both superficial and deep retinal layers (see Figure 5). The images also show extensive capillary closure in the affected area.
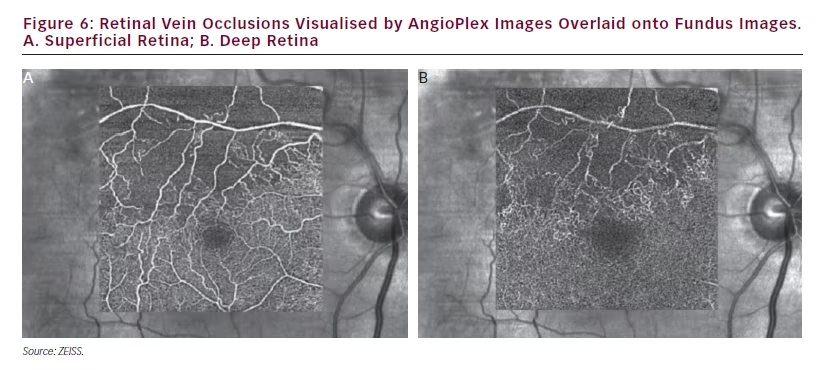
In a further case of vein occlusion (see Figure 6), AngioPlex OCT angiography images show the state of retinal perfusion is likely to be impaired due to the degree of capillary closure and apparent vascular damage. It is interesting to note that the larger blood vessels in the superficial layer can still be seen in the deep retina. The reason for this is that light that passes through the superficial vessels that overlay the deeper retina is affected by the motion in the superficial vessels, which causes the signals from the deeper retina to be affected, and leads to these bright shadow artifacts.23
Age-related Macular Degeneration
In AMD, AngioPlex OCT angiography can be used to demonstrate and monitor CNVs. The images produced are more similar to ICGA than to
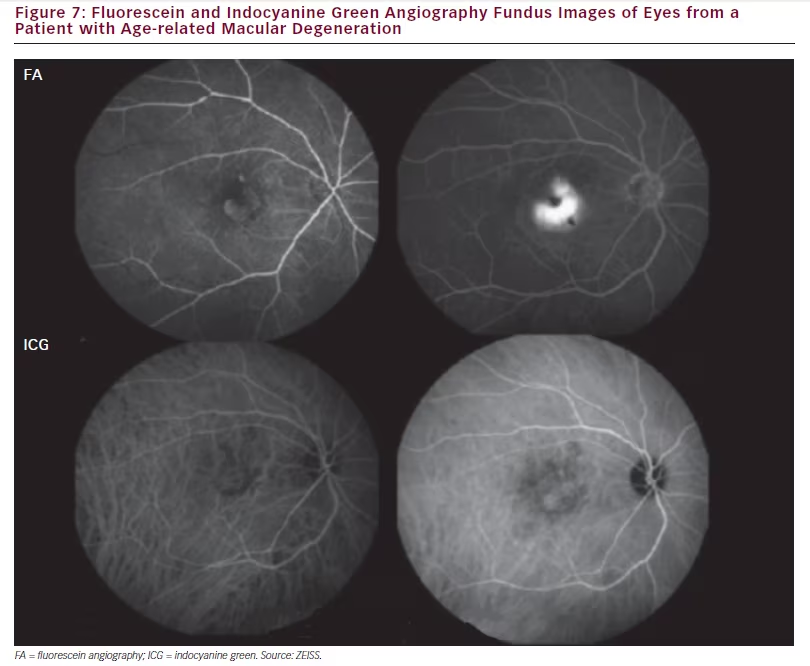
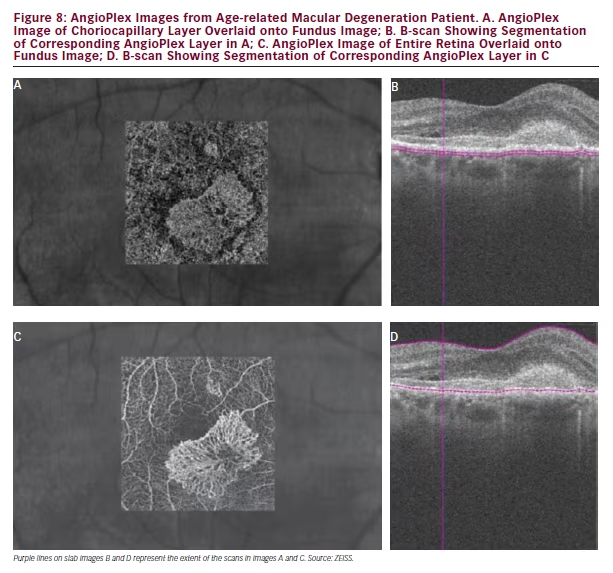
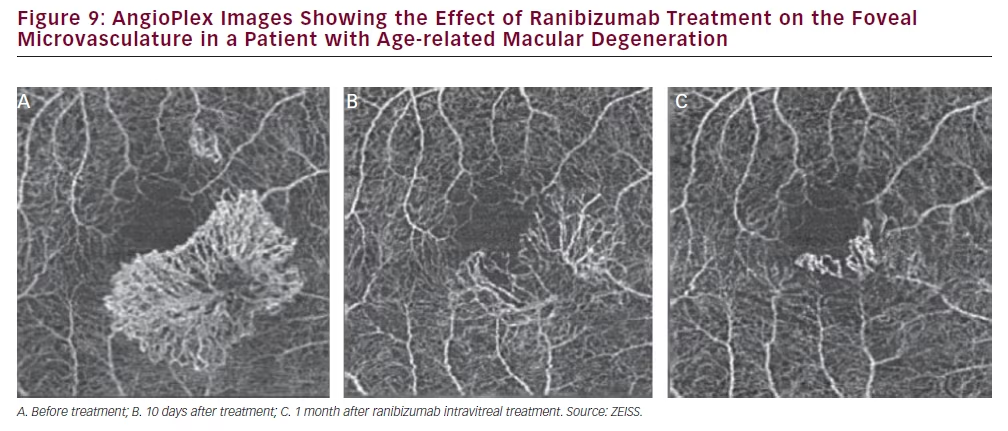
FA since the anatomy of the lesions and not the leakage are visualised (see Figure 7). AngioPlex also allows choriocapillary structures to be observed where ICGA does not. Figure 8 shows the choriocapillaris and the full retinal vasculature in a patient with AMD and a neovascular net is clearly visible. This net is likely to leak exudate and cause oedema and vision loss in the affected area. It should be noted that the apparent position of these vascular features can be affected by shadow effects of other structures such as membranes at the same position and so images should be interpreted with care. Figure 9> shows images from the same patient before, 10 days after and 1 month after ranibizumab treatment and the neovascular net appears to have diminished markedly. This technology may therefore provide an improved understanding on the effects of ranibizumab and other treatments on macular vasculature in AMD and various other ophthalmic diseases.
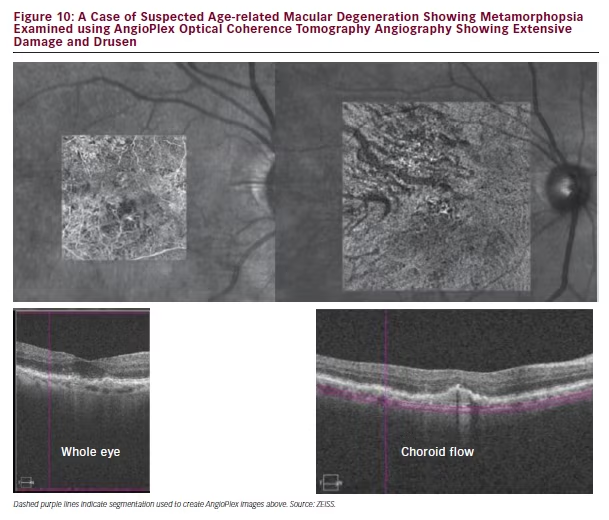
In a case of suspected AMD showing metamorphopsia, the patient was first examined using AngioPlex OCT angiography. Images of the whole eye and choroid flow beneath the retina revealed extensive areas of damage and drusen (see Figure 10). This justified further investigation using FA, which indicated CNV. AngioPlex OCT angiography could therefore be used as an initial and rapid means of examining ophthalmic conditions and the images could be used to decide whether invasive investigations using FA or ICGA are necessary.
Pathological Myopia
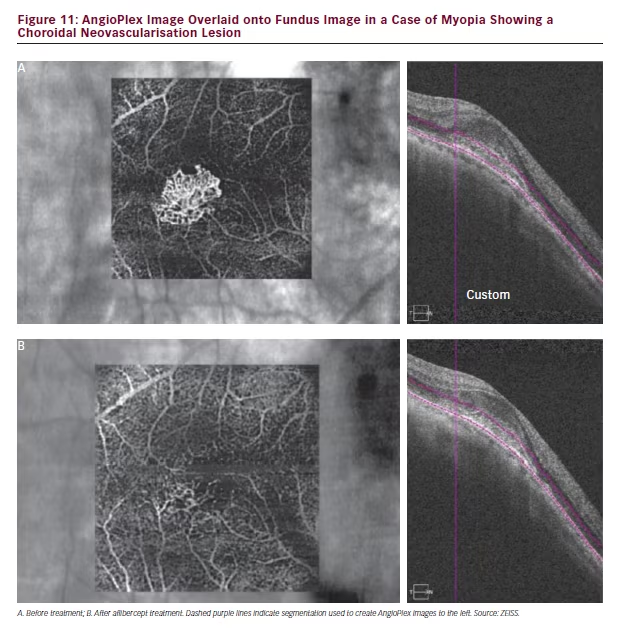
In a patient with myopia resulting from suspected CNV, AngioPlex OCT angiography revealed a microvascular net structure at the site of the lesion (see Figure 11). This feature, however, was not visible on the structural B-scan. Treatment with intravitreal aflibercept was seen to have markedly reduced this structure when scanned 1 week later. This finding further indicates that this technology can be used to both diagnose choroidal disease and monitor responses to treatment.
Optic Nerve
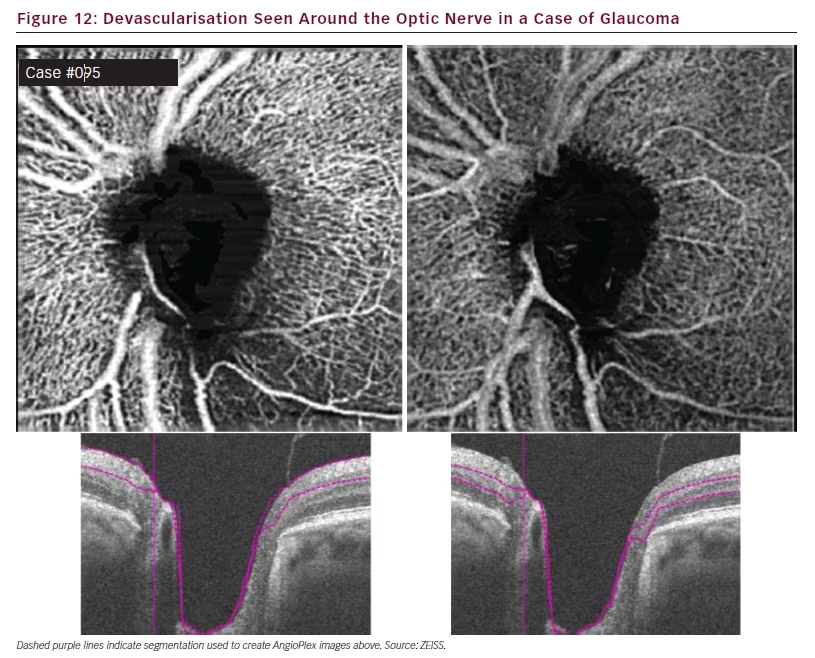
AngioPlex OCT angiography also has a highly novel application in the exploration of the vasculature of the optic nerve. In normal individuals such imaging shows a homogenous distribution of the vasculature around the disc in the superficial and deep retinal layers.24 In cases of glaucoma, however, the parts of this vasculature can be missing, producing prominent gaps. This feature can also be clearly seen in slab images (see Figure 12). Darkened regions around the disc indicate areas of poor perfusion and potential damage and this can be correlated with retinal nerve fibre layer defects.
Discussion and Conclusions
areas where they are interrupted by pathological processes. The high-quality images produced by this system enable the identification of capillary closure and neovascularisation and possible detection of microaneurysms and deep nets. Additional experience and development of new quantification techniques may allow future assessment of the ratio of dilatations in venules and arterioles in the fovea. OCT angiography can also penetrate haemorrhages but this depends on their thickness. The method can also identify non-perfused retinal areas better than FA due to the high contrast between vessels and surrounding retina. The system may additionally enable the investigation of ophthalmic tumours but that may depend on their size and level of pigmentation that would inhibit light penetration. Such illumination can be a concern when the retina is very thick and when capillary flow is slow: which is also a limitation of FA. Information on blood–retinal barrier leakage is, as yet, missing with this technique but with further development this may become apparent. The leakage occurring in conditions, such as cystoid macular oedema, cannot yet be defined but more data are needed. It is currently not possible to quantify capillary flow with this technology, this measurement would be valuable to the ophthalmologist and the addition of such a capability may be a future development.
The AngioPlex OCT angiography system is new and provides hitherto unknown ability to explore the retinal microvasculature. It also creates novel opportunities to examine deep retinal and choroidal layers and the optic nerve and extend previous work.25 Such ability will enable visualisation and potentially increased understanding of retinal disease. The higher level of image detail, ease and rapidity of use and improved patient experience will most likely result in the method replacing FA and ICGA in many cases for evaluation of retinal and choroidal vasculature. The system is available and has become the first angiography technology to be granted US Food and Drug Administration 510(k) clearance for clinical use.26