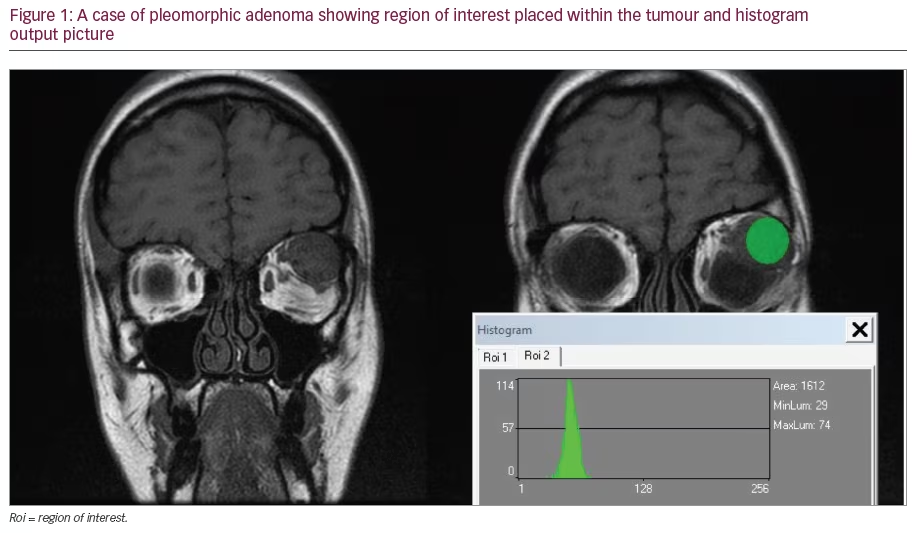
Evaluation and monitoring of many ocular diseases require the visualization of vitreal, retinal, and subretinal microscopic structures. Optical coherence tomography (OCT), first reported in 1991,1 is an interferometric tool for 3D imaging of objects hidden in scattering media such as the human eye. With spatial resolution of just a few microns, this non-invasive imaging system has been rapidly adapted for various medical applications, especially in the field of ophthalmology.
Evaluation and monitoring of many ocular diseases require the visualization of vitreal, retinal, and subretinal microscopic structures. Optical coherence tomography (OCT), first reported in 1991,1 is an interferometric tool for 3D imaging of objects hidden in scattering media such as the human eye. With spatial resolution of just a few microns, this non-invasive imaging system has been rapidly adapted for various medical applications, especially in the field of ophthalmology.
The revolutionary impact of OCT imaging systems on our understanding of ocular pathological processes has encouraged a close collaboration between engineering and ophthalmic experts. These academic and commercial collaborative efforts have resulted in a wave of OCT-related inventions. The most visible example is the classic time-domain OCT (TDOCT) technology. TDOCT was first commercialized in the mid-1990s and culminated in 2002 in the Stratus-OCT imaging system (Carl Zeiss Meditec, Dublin, CA), which is now a common fixture of most modern ophthalmic clinics.
Two more recent technologies—ultrahigh-resolution OCT (UHROCT) and spectral-domain OCT (SDOCT)—have further improved on the image quality of TDOCT systems. UHROCT utilizes ultra-broadband with light sources to provide nominal axial resolutions of 2–3μ (compared with 8–10μ in Stratus OCT), which reveals retinal morphology in more detail.2 However, since UHROCT requires femtosecond lasers or other very expensive light sources, it has not been widely used as a commercial/clinical OCT system. SDOCT technology, on the other hand, exploits the relatively compact, inexpensive, and low-maintenance super-luminescent diodes as its light source. While offering moderate improvement in axial resolution (~5μ), current commercial SDOCT systems provide a high scanning speed—50 times faster than TDOCT and 100 times faster than original time-domain UHROCT imaging systems.2With limited eye movement during such rapid scans, SDOCT provides superior image quality with reduced motion artifacts compared with TDOCT. The advantages of faster scans include but are not limited to the preservation of retinal topography, larger imaging field of view, greater sampling density, and improved correlation of the projected image sets to the fundus features.3
Utilization of SDOCT for the detection of a variety of ocular pathological features in retinal, glaucoma, or corneal studies has been reported in the recent literature. For example, to follow the progression of age-related macular degeneration (AMD), our laboratory has studied SDOCT imaging for the measurement and monitoring of macular thickness, retinal pigment epithelial (RPE) layer, dry drusen, and geographic atrophy (GA).4 We have also found SDOCT to be an invaluable tool for the visualization and detection of choroidal neovascularization (CNV), cystoid macular edema (CME), subretinal fluid, and other subretinal structures in eyes with neovascular AMD.5 A representative example delineating the 3D structure of the CNV, neovascular complex and subretinal fluid of an AMD patient5 is shown in Figure 1. Indeed, tomographic and radiometric variability in drusen appearance has also been reported using UHROCT6 and SDOCT7 systems.
We leave the study of the state-of-the-art common ‘table-top’ SDOCT ophthalmic applications to the recent survey papers4 and special-issue journals.8 In what follows in this paper, we review some emerging developments in SDOCT technology that we believe may lead to paradigm shifts in the way in which ophthalmic imaging is performed for research and clinical applications.
Mobile Spectral-domain Optical Coherence Tomography
Interferometric-based imaging systems such as SDOCT are commonly considered as stationary table-top tools for examining alert adult patients. We predict that the commercialization of mobile SDOCT systems will expand the application spectrum to aim at patients with uncontrollable motion or pediatric imaging. The first commercialized mobile SDOCT scanner (Bioptigen Inc., Research Triangle Park, NC) provides a light hand-held imaging probe that can be maneuvered independently of the main body.9
Aside from the obvious applications in imaging small animal eyes,9 we have recently reported on two novel applications of portable SDOCT systems. One application is the evaluation of the spectrum of foveal architecture in pediatric ocular albinism (OA).10 Figure 2A shows the summed voxel projection(SVP) of an OA infant10 (SVP is a 2D image analogous to the fundus photograph created by axially collapsing the B-scans3). Figures 2B and 2C are two corresponding B-scans showing the abnormal elevation of the optic nerve and the absence of the foveal depression, respectively. In another report,11 we demonstrated the comparable quality and performance of the hand-held and table-top SDOCT systems in evaluating macular hole and CMO in adults. For infants, we reported on the application of hand-held SDOCT in evaluating the extent of retinal pathology from shaken-baby syndrome in the pre-, mid-, and post-surgery stages.
Spectral-domain Optical Coherence Tomography for Intraoperative Use in Vitroretinal Surgery
Vitreoretinal surgeons have long relied on the optical stereo microscope to visualize the surgical field. Even with recent design improvements, limitations to intraoperative visualization and accurate localization of very small or thin translucent structures within the eye (e.g. the posterior hyaloid) remain a major factor restricting the capabilities of ophthalmic surgeons. Moreover, current imaging modalities do not provide realtime cross-sectional images of the change in location of a surgical instrument relative to tissue or of tissue deformation during surgery. Such imaging feedback could be important in judging whether to continue with, stop, or change a specific maneuver. A robust SDOCT surgical microscope could provide a base for significant advances in ocular surgery and other branches of microsurgical intervention. Aside from the surgical room, such an imaging modality may also be useful for in-clinic subretinal drug-delivery applications.
We have conducted preliminary surgical experiments on cadaver animal eyes using the hand-held retinal SDOCT scanner from Bioptigen, Inc. Our experiments show that surgical tools may be readily visualized on SDOCT B-scans, for which realtime display is critical. An illustrative example showing the diamond-shaped metal tip of a 20-gauge micro-vitreoretinal (MVR) blade and its trajectory on SDOCT images is shown in Figure 3. This and other technical breakthroughs are encouraging factors for the development of surgical SDOCT system and accompanying software.
Functional/Targeted Spectral-domain Optical Coherence Tomography Imaging
Unlike many other medical imaging modalities with functional adjuncts, such as computed tomography (CT) and magnetic resonance imaging (MRI), common ophthalmic use of SDOCT has been restricted to structural imaging. In structural imaging, regardless of the metabolic or physiological dynamics, the pixel value (brightness) of the captured SDOCT images directly correlates to the light-scattering properties of the imaged tissues. For example, on SDOCT images of the retina, the blood vessels and the photoreceptor layer (PRL) both appear dark compared with surrounding tissue layers. In many cases, based on the assumed normal shape, size, or location, it is only the educated guess of the ophthalmic expert that distinguishes one subretinal structure from another. In the following, we review two new functional SDOCT imaging modalities with a potentially wide spectrum of diagnostic application.
Doppler Spectral-domain Optical Coherence Tomography for Blood-flow Imaging
Ultrasonic Doppler imaging systems have long been used for characterization of valve function and plaque build-up in the cardiovascular system, as well as imaging the anterior segment of the eye. However, Doppler ultrasound does not have the resolution or depth of penetration needed to evaluate the retinal vasculature. Doppler OCT technology was first developed using TDOCT systems12,13 and was later utilized for retinal flow analysis.14 However, the slow data acquisition compounded with unavoidable patient head motion severely restricted the reliability of the data. In SDOCT systems, Doppler flow velocities are acquired much more quickly from the slope of the phase of the depthresolved scattered light signal over several repeated depth scans at the same lateral position.15 Cross-sectional retinal Doppler flow data from a living human patient acquired using a commercial SDOCT system are illustrated in Figure 4. Recent extensions to Doppler SDOCT are enabling complete 3D mapping of the retinal vasculature for the first time,16,17 with potential applications in monitoring diabetic retinopathy and other blinding diseases with a vascular component.
Polarization-sensitive Spectral-domain Optical Coherence Tomography for Retinal Pigment Epithelial Imaging
Another exciting technological advance in SDOCT is tissue-selective imaging capability. Recent reports on exploiting contrast agents to enhance the target selectivity of OCT systems are very promising.18,19 However, no report on in vivo human SDOCT ophthalmic imaging using contrast agents has yet been published. Polarization-sensitive (PS)-SDOCT is an alternative non-invasive tissue-selective imaging tool. PS-SDOCT systems resolve the accumulated path-length difference to sample reflections into orthogonal vector dimensions and plot the results as images of either net retardance or of the fast axis orientation. Hitzenberger et al. have recently pointed out that since the RPE layer of the retina appears to depolarize light, this layer appears distinct from other tissue layers in PS-SDOCT images of retardance and fast axis.20 We have recently shown that this demarcation can be quantified from the standard deviation of the fast-axis orientation,21 as illustrated in Figure 5. Therefore, we believe that PS-SDOCT is a promising tool for AMD evaluation, in which tracking RPE degeneration and dislocation is of great interest. Considering other recent applications in glaucoma diagnosis22 and the recent development of faster and more sensitive systems,21 it is expected that PS-SDOCT will find more practical applications in large-scale studies.
Concluding Remarks
In this paper, we have reviewed alternate designs for the SDOCT technology with non-conventional ophthalmic applications. Indeed, these were just a few representative examples of the exciting new discoveries in the field of ophthalmic SDOCT imaging. Other examples include recent reports on the incorporation of SDOCT and adaptive optics (AO) technologies23 to reduce the eye’s aberration effect and improve resolution. Another hardware modification comes from exploiting light sources that operate at the 1,050nm wavelength (in place of the current 850nm) to improve penetration depth.24 On another front, novel software-based image-processing systems have shown potential in improving the resolution of SDOCT systems without increasing the production cost.25
Finally, we note that these and other recent advances in OCT design (e.g. using Fourier domain-mode locking lasers)26 will significantly improve image acquisition speed. The avalanche of data created by these novel technologies is too large to be fully analyzed and interpreted by ophthalmic experts. Therefore, more research effort needs to be allocated for the development of automatic or semi-automatic image analysis systems that expedite the decision-making process of physicians.27 Moreover, the creation of standardized data management protocols (e.g. digital imaging and communications in medicine [DICOM]) to safely handle, store, and transmit OCT data is essential for large-scale multi-institutional studies. We expect that these and other upcoming technological advances will expand the ophthalmic applications of OCT technology, vastly improving our ability to study ocular disease processes.