Ab interno trabeculectomy with the Trabectome® (NeoMedix , Tustin, CA, US) is a minimally invasive glaucoma surgery (MIGS) modality that increases conventional outflow over 6 clock hours through a single incision.1 The tip of the Trabectome handpiece generates plasma that ionizes and ablates the trabecular meshwork (TM), a mechanism that is fundamentally different from cautery. The primary mode of action is enhancement of conventional outflow along the physiologic route and in this regard is similar to TM bypass microstents,2,3 which produce a more segmentally limited outflow.4 The growing prevalence of glaucoma,5,6 along with increasing longevity and the desire to maintain physical, social, and occupational fitness, underscores the need for effective and well-tolerated glaucoma surgeries. Trabeculectomies and tube shunts, while effective, are associated with serious risks that amounted to 74 % of trabeculectomies and 27 % of tubes needing manipulation. Thirty-nine percent of trabeculectomies had early and 38 % had late vision-threatening complications (total of 77 %) compared with tube shunts with 22 % early and 36 % (total of 58 %) experiencing vision-threatening complications during 5 years follow up.7 Other patient populations and surgeons have reported more favorable results, but these studies were not nonrandomized controlled.8,9 As MIGS are standardized with predictable surgeon factors, they can be well combined with cataract surgery and allow implantation of advanced intraocular lenses.
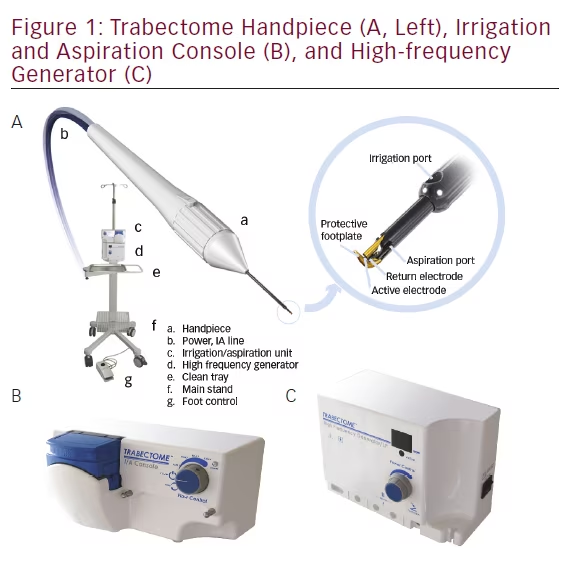
The Trabectome (see Figure 1) was granted US Food and Drug Administration (FDA) approval in the US in April 2004 for use in adult as well as pediatric glaucoma as the only device specifically approved for this population.10 The tip design was updated in 2012 with a more beveled sleeve to allow easier entry and larger irrigation ports for improved chamber stability. Use of viscoelastic prior to Trabectome surgery was discouraged and removed from the package. The Trabectome has gained widespread international adoption in the decade following its introduction.11,12 The current commercial device was developed in conjunction with the NeoMedix Corporation, who continues to distribute and market the device platform.
Trabectome Patient Selection
The original studies of Trabectome surgery included an indication of glaucoma with an angle open to at least 20° and excluded narrow angles and neovascular glaucoma.13 Subsequent studies have shown that Trabectome may also be effective in eyes with a narrow to very narrow angle.11 We
have operated on a wide range of secondary glaucomas, including following trauma, scleral buckle, uveitis,14 and failed trabeculectomies12 with successful lowering of intraocular pressure (IOP). The only remaining contraindications include active neovascular, active uveitic, or glaucoma due to increased episcleral pressure, since none of these would be thought to respond sufficiently to TM ablation.
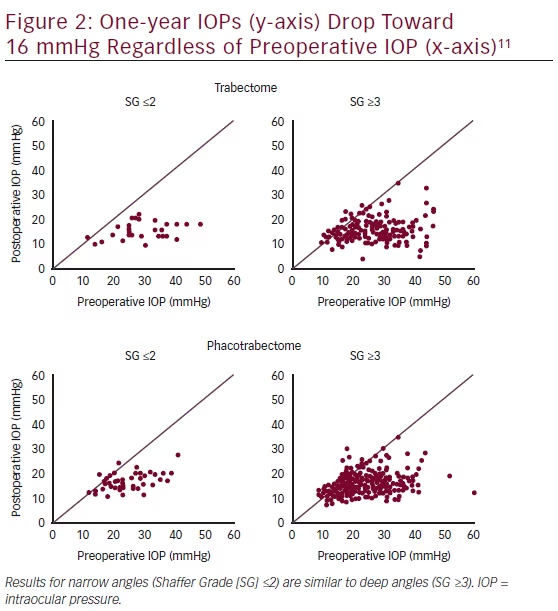
Since successful Trabectome surgery frequently lowers the IOP into the midteens with relatively little influence of baseline IOP as evidenced by the single IOP dot plots11,12 (see results section, Figure 2), there does not appear to be an ideal baseline as patients with a higher preoperative IOP have a larger reduction that can be estimated with a formula as described below. The theoretical limit to postoperative IOP is the episcleral venous pressure. The severity of glaucoma does not appear to negatively influence IOP outcome.12 On the contrary, glaucomas with a more diseased TM and a higher relative glaucoma disease index show a larger IOP drop (unpublished data). Rather than focusing on the absolute decrease in IOP, some surgeons use a reduction of medication dependence as an indication, while others use Trabectome to treat ocular hypertension.
Trabectome as the Initial Glaucoma Surgery
No randomized controlled trial has formally compared Trabectome surgery to trabeculectomy or tube shunt surgery. Nonmatched, retrospective comparisons have reported conflicting results. Francis et al., found that while the average IOP was lower after trabeculectomy at 1 year, the success rate was 95 % in patients who underwent phacotrabectome surgery compared with 83 % in the phacotrabeculectomy group using the same success criteria (IOP <21 mmHg and no secondary surgery and IOP reduction ≥20 %).15 Mosaed et al. found a range of 55 % to 95 % after Trabectome only compared with 86.5 % after trabeculectomy only.16 Jea et al.17 found a considerably lower success rate for Trabectome surgery.
The main reasons for selecting Trabectome as a first choice over trabeculectomies and tube shunt surgeries are that the procedure is faster than penetrating surgery, has a more routine postoperative course with fast visual recovery, and significantly fewer complications. The complication profile is not unlike that of cataract surgery with the exception of postoperative hyphema. Despite the high frequency of hyphema,17 a total of only three cases have been reported where intervention was necessary.18,19 Anticoagulants such as aspirin, coumadin, or antiplatelet agents can be safely continued. Since the surgery does not involve the conjunctiva, there should be no significant effect in cases when penetrating surgery is needed after unsuccessful Trabectome. Indeed, one of the first Trabectome investigations analyzed trabeculectomy after a failed Trabectome and found no difference in IOP or complications compared with a group undergoing primary trabeculectomies.20 Trabectome surgery has also demonstrated success without same session cataract surgery, after failed trabeculectomy,12 after tube shunts,21 canaloplasty, laser trabeculoplasty,22 and other complex glaucomas that include trauma, scleral buckles,14 and strabismus surgery.
Patient Considerations
Trabectome is ideal for active patients who need predictable visual recovery, do not want to have the longer-term risks associated with a bleb from trabeculectomy, or who want to wear contact lenses. Although predicting individual outcomes can only go so far, a new Trabectome surgery calculator,23 based on data of more than 600 patients in a matched comparison, can help to guide the surgeon in establishing preoperative expectations. The approximate IOP reduction can be predicted with the formula -13.54+0.73*(phaco; yes:1, no:0)+0.73*(baseline IOP)+0.59*(secondary open-angle glaucoma [SOAG]; yes:1, no:0)+0.03*(age)+0.09*(medications). This can aid in determining if the likely final IOP is within the patient’s target range or if eye drops will be needed.
Cataracts can be removed in the same session. Since only a standardized clear corneal incision is used with a predictable surgeon factor, astigmatic
lenses can be safely implanted. Other situations that may see greater benefits from choosing Trabectome are patients in whom frequent postoperative visits and potential manipulations (i.e. reforming a flat anterior chamber) would present a great challenge, and patients, such as high myopes, who are at an increased risk for complications of hypotony including hypotony maculopathy, choroidals, and a flat chamber that are unlikely following Trabectome surgery compared with penetrating glaucoma surgery. There is limited published information on using the Trabectome for glaucoma of childhood24 although it is easier to perform than the standard: goniotomy. The latter requires vast experience in a nonpressurized anterior chamber, a proficient assistant who can counterrotate the eye and assist with visualization, and the ability to estimate the depth of the goniotomy knife’s penetration into the TM. By contrast, the footplate on the Trabectome handpiece and pressurized anterior chamber would make for a more straightforward removal of TM.
Exclusion Criteria
In order to assure a stable gonioscopic view of the angle, the patient must be able to rotate their neck or entire axial core. The view must be confirmed to be clear of any corneal opacity. As noted above, active neovascular, active uveitic, or glaucoma due to elevated episcleral pressure are thought to have poor outcomes with TM ablation. Since late recurrent hyphemas have been reported,25 individuals who engage in frequent activities that increase episcleral venous pressure such as playing wind instruments or upside-down positioning have to be cautioned.
Review of Technique
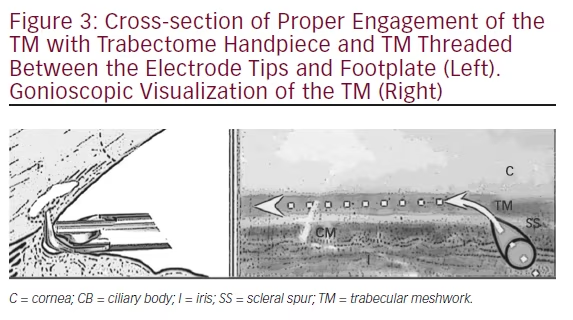
Practicing visualization of the TM is a crucial step in preparing for initial Trabectome surgeries (see Figure 3). In fact, a model has been published that allows for gonioscopic visualization of donor eyes.26 Reviews are available on the topic.27

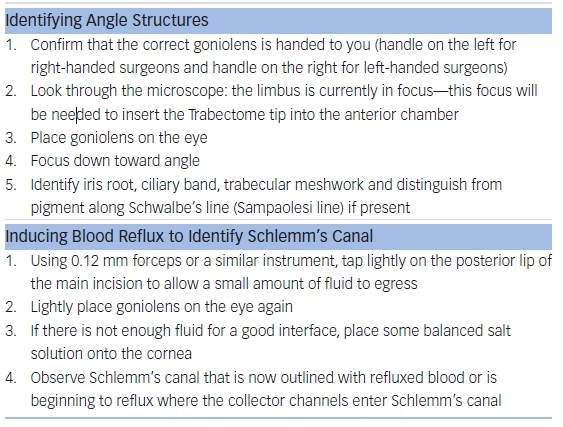
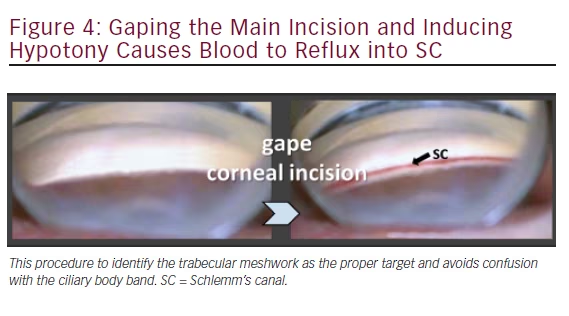
A paracentesis is fashioned as for phacoemulsification and preservativefree lidocaine is injected. No viscoelastic is required. The 1.6 mm main wound can be made more anterior (approximately 2 mm anterior to the limbus) to avoid iris prolapse as well as corneal striae during ablation. The patient’s head and microscope are rotated 30–40° each until adequate visualization of the angle is achieved with the goniolens (see Table 1). If the TM cannot be visualized because of minimal pigmentation, then the posterior lip of the incision can be depressed to allow a small amount of fluid to egress. The reflux of blood will highlight Schlemm’s canal (see Figure 4).
The TM is engaged slightly toward the left and by pointing up at a 45° angle. It is important to resist pushing outward during ablation so as to not damage the collector channels. Ablation is started at 0.8 mW and titrated up if necessary.28 Ablation of up to 90° in both directions can be performed. A viscoadaptive substance can then be injected to fill about 20 % of the anterior chamber in order to leave a crescent of viscoelastic tamponading the ablation arc to minimize postoperative hyphema (see Table 2). In narrow angles with synechiae, in order to gain access to the TM for ablation, goniosynechiolysis can be performed using the smooth base plate of the handpiece.29


Patient Management and Postoperative Care
Patients should be warned that blurry vision is expected and usually lasts 3 to 10 days (see Table 3). With dense postoperative inflammation or hyphema, the visual acuity on postoperative day 1 can be as low as counting fingers. Patients may also observe that their vision is worse in the morning and gets better upon rising from shifting hyphema. Unlike with traumatic hyphemas, these patients do not require patching, atropinization, or bed rest as it will resolve spontaneously. Routine postoperative drops are similar to cataract surgery and include a fluoroquinolone, prednisolone acetate, and pilocarpine 1–2 % four times daily. The prednisolone should be tapered weekly. Pilocarpine can be tapered to three times per day after 1 month and stopped after 2 months. Glaucoma medications can be partially or completely stopped depending on the severity of the glaucoma and postoperative IOP.
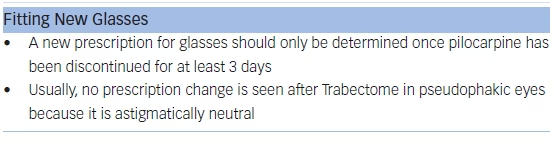
Light aerobic exercise without strenuous lifting or squatting is acceptable within a few days after surgery. As discussed above, strenuous activity and inversion can trigger recurrent hyphema. New glasses should not be dispensed until after pilocarpine has been stopped.


Outcomes
In most cases, the IOP will be lowered into the midteens with a pressure reduction between 30 and 40 %.1 Although it is difficult and not accurate to combine various studies with different indications and populations, an overall average of outcomes can give some idea of the expected outcome to compare with other options. Whether or not it is combined with phacoemulsification, Trabectome has been shown to frequently lower the IOP to a final average around 16 mmHg (see Figure 2) while decreasing the number of medications by one.17,30–32
There are limited data to help determine the success rate of the procedure, with few studies analyzing this rigorously. One definition of success used was final IOP ≤21 mmHg with a 20 % decrease from baseline while avoiding reoperation. For standalone Trabectome, this occurred in approximately 70 %33,34 of cases by the end of 1 year but then in one study fell as low as 22 % by year 2.35 For phacoemulsification with Trabectome, it was approximately 90 %15,33 after 1 year and was 80 % at 2 years.36
Two prospective studies indicate that pigmentary glaucomas have a better response to Trabectome than does primary open-angle glaucoma.19,34 The main difference between the two groups was success rates, with the pigmentary glaucomas having a 15–20 % higher success rate.19,34 The improved results seen may correspond to the bulk of outflow resistance being localized only in the TM in pigmentary glaucomas.37
Complications
Trabectome has a significantly better safety profile compared with trabeculectomy and tube shunts, since there is nearly no risk for hypotony, wound leak, shallow anterior chamber, and choroidals, or the long-term risk for endophthalmitis and hardware erosion.38 The most common side effect of Trabectome is transient hyphema indicating reflux from a patent drainage system. It almost always resolves on its own within days and delayed-onset hyphema is rare.39–41 Other reported side effects include peripheral anterior synechiae in up to 24 % of 37 patients.13 There are also case reports of cystoid macular edema, vitreous block, cyclodialysis cleft, endophthalmitis, and suprachoroidal hemorrhage.13,38,42 Complication rates in these articles do not exceed 1 %.17,43
Additional Considerations
Current annual medical spending for glaucoma accounts for about $2.9 billion in the US.44 As reported by a cost analysis in Ontario, Canada, after 6 years Trabectome saved C$279.23 over monodrug and $2,424.71 per patient versus tridrug therapy.45 Trabectome was more economical than iStent but less than endoscopic cyclophotocoagulation. Combination of Trabectome with tube shunt implantation to achieve lower IOPs and a blunted hypertensive phase or repeated use of Trabectome for cases after failure is another area for further research and may be able to reduce medication dependence and IOP further while minimizing complications in eyes with multiple procedures.12
Conclusion
Trabectome surgery has been shown to be an effective option for lowering the IOP with a favorable side effect profile compared with trabeculectomy or tube shunt surgery. Since the first results in 2005, the indications have since expanded with successful outcomes in most types of primary and secondary open- and narrow angle-glaucoma. Perioperative considerations are similar to phacoemulsification with a similar speed of visual recovery. The major postoperative difference is the occurrence of hyphema, which can be reduced by leaving a crescent of viscoelastic to briefly tamponade the exposed collector channels. The IOP in successful cases is frequently lowered into the midteens while decreasing the number of medications by one. Less information is available on success rates, which are likely highest for pigmentary glaucomas.







