Introduction
In the recently updated Dry Eye WorkShop DEWS II report, dry eye is defined as a multifactorial disease of the ocular surface characterized by loss of homeostasis of the tear film, accompanied by ocular symptoms, tear film instability, hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities.1 Chronic, T cell–mediated inflammation of the
ocular surface and lacrimal glands plays a prominent role as signaling pathways, activated in a variety of cell types by stress to the ocular surface, upregulate proinflammatory proteins, contribute to further damage, and lead to a self-perpetuating inflammatory cycle.2–4 Dry eye is often associated with ocular discomfort, which typically worsens in the evening.5 In addition, intermittent blurred vision often develops from corneal surface irregularities and tear film abnormalities though normal visual acuity may be measured on a standard eye chart.1,6 Impaired visual function can significantly impact vision-dependent activities associated with social and physical functioning (e.g., reading, driving at night) and has an adverse impact on workplace productivity and overall quality of life.7–9 Patients with severe dry eye have reported reduced quality of life comparable to that of moderate to severe angina.10
Dry eye disease is a global public health concern. Prevalence increases with age and current estimates suggest that 5–50% of the global population is affected by dry eye.11 In recent years, the prevalence may have increased as a result of the aging population and more frequent use of contact lenses, computers, smartphones, and tablets.12–14 In a recent meta-analysis of prior reports of dry eye disease prevalence in visual display terminal workers, the prevalence of dry eye ranged from 9.5–87.5% across 16 studies, with the vast differences attributed to differences in criteria for defining dry eye.15
Symptomatic dry eye disease can present initially without evidence of ocular surface damage or changes in tear production.7,8,16 Discordance between diagnostic tests and patient-reported symptoms17,18 poses a challenge for accurately diagnosing and treating patients with dry eye. Several therapeutic options are currently available in the United States to patients with dry eye. Cyclosporine ophthalmic emulsion 0.05% (Restasis®, Allergan plc, Dublin, Ireland) was approved in 2002 to increase tear production in patients whose tear production is presumed to be suppressed owing to ocular inflammation associated with keratoconjunctivitis sicca.19 In clinical trials, cyclosporine ophthalmic emulsion resulted in greater improvements in tear production, corneal staining, and dry eye symptoms compared with vehicle.20,21 In 2016, lifitegrast ophthalmic solution 5% (Xiidra®, Novartis International AG, Basel, Switzerland), was approved for the treatment of the signs and symptoms of dry eye disease.22
Given the increasing use of visual display terminals and their impact on dry eye, the present study was conducted to evaluate the efficacy of cyclosporine ophthalmic emulsion 0.05% in dry eye subjects with associated ocular discomfort when engaging in frequent electronic visual tasking.
Methods
Study design and eligibility
This multicenter, open-label study (ClinicalTrials.gov identifier NCT02554981) was performed in compliance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice, Health Insurance Portability and Accountability Act. The study protocol was reviewed by the Alpha Independent Review Board, San Clemente, California. All subjects provided written consent before initiating any study treatment.
Subjects were eligible if they were between 18–55 years of age with a history of a dry eye disease in both eyes and dry eye symptoms (≥2 on the Ora CalibraTM Ocular Discomfort and 4-Symptom Questionnaire) when using video display terminals. Key inclusion criteria also included ocular surface staining (≥2 and <4 in at least one corneal region, central corneal staining ≥2 on the Ora Calibra scale), conjunctival redness (≥1 on the Ora Calibra scale), and Schirmer score <10 mm in at least one eye, and total Ocular Surface Disease Index© (OSDI©) >12. A diagnosis of Sjögren’s syndrome was not required for inclusion. Key exclusion criteria included active ocular infection, use of Restasis® within the prior 90 days, refractive surgery within 12 months, punctal occlusion, use of contact lenses, or use of systemic medication known to cause ocular drying that had not been used on a stable dosing regimen for at least 30 days and was expected to remain stable during the study period.
Study visits and interventions
The study consisted of five visits: visit 1 (day 0), visit 2A (day 0 ± 1–3 days, morning), visit 2B (10 ± 2 hours following visit 2A), visit 3 (1 month after study initiation), visit 4A (6 months after study initiation), visit 4B (10 ± 2 hours following visit 4A), and visit 5 (exit visit; 1–3 days after visit 4). Additional telephone contact was made between visits at day 14 and months 2, 3, 4, and 5 after study initiation. Subjects were queried about the average amount of time per day during the last week spent using a computer, television, or smartphone or playing a videogame. At visit 2B, subjects received a 6-month supply of cyclosporine ophthalmic emulsion 0.05% (Restasis®) and were instructed to instill one drop of the emulsion twice daily in both eyes for the duration of the study, beginning the evening of visit 2B. Artificial tears (Refresh Optive® Advanced, Allergan plc, Dublin, Ireland) were also provided for subjects to use as needed. At visits 2A and 4A, a diurnal blink device (Ora, Inc.), which is a validated electromyographic-based blink monitor, was fitted.23 This device monitors blink pattern and rate over a period of approximately 10 hours. During this period, each subject recorded electronic visual tasking using a daily diary to note activity at 30-minute intervals. Diaries were collected at visits 2B and 4B and the blink device was removed.
Primary efficacy assessments
The primary efficacy measures were ocular surface staining and visual function at month 6. Ocular surface staining was assessed by instilling 5 μL sodium fluorescein 2% solution and 10 μL lissamine green solution into the inferior conjunctival cul-de-sac of each of the subject’s eyes. Fluorescein staining was evaluated 3 to 5 minutes after instilling fluorescein, which allowed maximum fluorescence to be achieved, while lissamine green staining was evaluated 30 seconds after blinking following instillation of lissamine green; eyes were graded using a 5-point Ora Calibra Corneal and Conjunctival Staining Scale (0 [none] to 4 [confluent staining, denoted as severe], where half unit increments were permitted), respectively.24,25 Both vital dyes were used to assess both corneal and conjunctival staining: ocular surface staining was assessed as total grade (all corneal and conjunctival regions), total cornea grade (central, inferior, and superior regions), total conjunctiva grade (temporal and nasal regions), and each of the five individual regions.
Visual function was evaluated using a series of three separate tests—the Ora Calibra Menu Reading Test, IReST Reading Test, and Wilkins Rate of Reading Test. These tests evaluated the time taken to read a passage, the number of words read incorrectly, and font size.
Secondary efficacy assessments
Secondary efficacy measures of visual function and symptom assessments included tear film break-up time (TFBUT), interblink interval (IBI), Ocular Protection Index 2.0 (OPI 2.0), the Ora Calibra Ocular Discomfort Scale, the Ora Calibra Ocular Discomfort and 4-Symptom Questionnaire, and tear production assessed by Schirmer’s test score.
Subject-rated visual function was evaluated using the OSDI questionnaire (Appendix 1), which consists of 12 questions that assess ocular symptoms (light sensitivity, grittiness, pain/soreness), ability to perform visual-related tasks (blurry vision, poor vision, reading, night driving, computer/bank machine use, watching television), and the effect of environmental conditions (wind, low humidity, air conditioning). These were graded on a scale ranging from 0 (none of the time) to 4 (all of the time), and the total score was calculated as OSDI = [(sum of all scores for questions answered) x 100] / [(number of questions answered) x 4].10,26 The total OSDI scores were further categorized according to severity (mild >12, moderate >22, and severe >32), and the percentage of subjects was assessed in each category at baseline and after 6 months.27
The change in TFBUT from baseline to visit 5 (6 months) was assessed in the worse eye (at baseline). The mean change in IBI from baseline to visit 5 was assessed by measuring the time (seconds) between blinks in the worse eye using the blink device. An increase from baseline in the IBI indicates a worsening in visual function (more frequent blinks). The OPI 2.0 system implements fully automated software algorithms that provide a real-time measurement of corneal exposure (break-up area) for each IBI during a 1-minute video and was assessed at baseline and visit 5.28
Subjects were asked to subjectively grade their ocular discomfort for each eye using a 5-point Ora Calibra Ocular Discomfort Scale (0 [no discomfort] to 4 [severe]). The severity of dry eye symptoms was assessed using the 6-point Ora Calibra Ocular Discomfort and 4-Symptom Questionnaire; five ocular symptoms, overall discomfort, burning, dryness, grittiness, and stinging, were graded on a scale from 0 (no pain) to 5 (worst pain).24 As part of the visual function assessments, ocular discomfort scores were also collected following Wilkins Rate of Reading Test. In addition, diurnal dry eye symptoms were assessed in the morning and evening on study days that blink rate was measured (visit 2 and visit 4).
Schirmer’s test (without anesthesia) was performed to measure tear production. Sterile Schirmer’s test strips (Tear Flo, Rose Stone Enterprises, Alta Loma, CA, USA) were placed in the lower cul-de-sac at the junction of the temporal and central one-third of the lower eyelid margin. Subjects were instructed to close their eyes for 5 minutes, then the strip was removed, and the length of the moistened area measured.
Safety assessments
Safety measures included monitoring adverse events (AEs), slit lamp biomicroscopy, and visual acuity. The Early Treatment of Diabetic Retinopathy Study chart was used to assess visual acuity.29 An increase of ≥0.22 in LogMAR score was judged as clinically significant and recorded as an AE.
Statistical analyses
For all analyses, the worse eye and fellow eye were identified for each subject. The worse eye was defined as the eye with the highest central corneal fluorescein staining at baseline (visit 1). If both eyes had similar central staining at baseline, the right eye was considered the worse eye for analysis. Efficacy analyses were performed on the intent-to-treat (ITT) population (all subjects receiving one dose of study drug) with observed data only by worse eye. The average of both eyes was used as a secondary analysis. The per-protocol population included subjects who completed the study without any major protocol deviations. The safety population was the same as the ITT population.
The primary efficacy endpoints were the change in fluorescein staining and lissamine green staining as graded by the Ora Calibra scale, and change in the Ora Calibra Menu Reading Test, IReST Reading Test, and Wilkins Rate of Reading Test at visit 5 (6 months) compared with baseline. Secondary efficacy analyses included OSDI individual questions and overall score, the change in TFBUT, blink rate (IBI), OPI 2.0, Ora Calibra Ocular Discomfort Scale, Ora Calibra Ocular Discomfort and 4-Symptom Questionnaire, and Schirmer’s test score at visit 5 compared with baseline. Quantitative variables were summarized using number of observations (n), mean, standard deviation (SD), median, minimum, and maximum. For data collected over time, the observed data at each visit were summarized and change from baseline (visit 1) was summarized at visit 5. Safety measures were summarized using counts and percentages. Paired t-tests were used to determine whether changes from baseline indicated any significant improvements for all efficacy analyses, with p<0.05 level of significance. Statistical programming and analyses were performed using SAS® Version 9.2 (Cary, NC, USA). Data and associated protocols are available upon request to Allergan plc.
Results
A total of 51 subjects were enrolled between July 21, 2015, and March 24, 2016, in two centers in the United States; one subject discontinued the study before dosing. The mean ± SD age was 46.3 ± 8.3 years and the majority of subjects were female (82%) and white (72%). Overall, 46 (90%) subjects completed the study (per-protocol population). Three subjects withdrew consent, one was discontinued due to an AE unrelated to study drug, and one subject was withdrawn for administrative reasons.
Efficacy outcomes
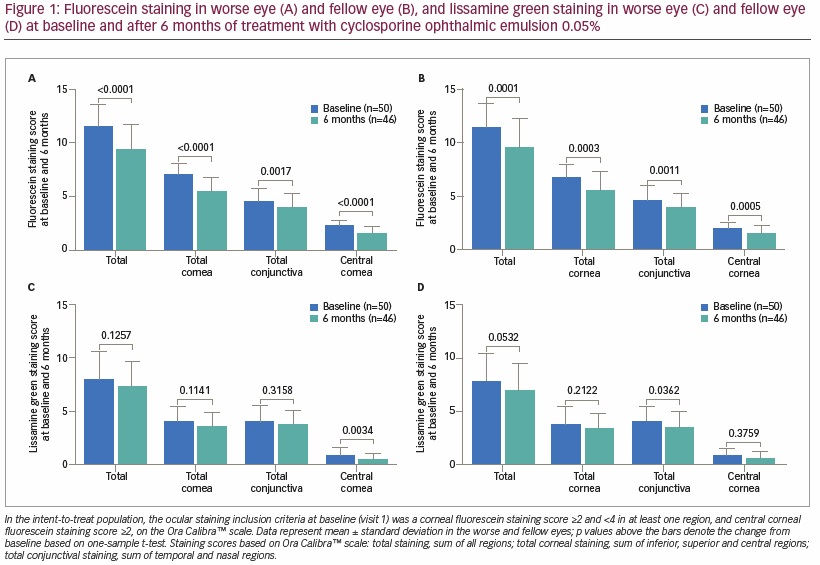
At baseline, mean ± SD total ocular surface fluorescein staining was 11.6 ± 2.0 in the worse eye and 11.2 ± 2.2 in the fellow eye. Significant reductions in fluorescein staining were observed in total ocular surface areas of both the worse eye (-2.1 ± 2.4; p<0.0001) and fellow eye (-1.6 ± 2.6; p=0.0001) after 6 months of topical cyclosporine (Ora Calibra scale; Figures 1A and B). In addition, significant reductions were also observed after 6 months of cyclosporine in fluorescein staining in total cornea (-1.5 ± 1.5 [p<0.0001], -1.0 ± 1.8 [p=0.0003]), central cornea (-0.8 ± 0.7 [p<0.0001], -0.5 ± 0.8 [p=0.0005]), inferior cornea (-0.4 ± 0.8 [p=0.0008], -0.3 ± 0.8 [p=0.0057]), and total conjunctiva (-0.6 ± 1.2 [p=0.0017], -0.6 ± 1.2 [p=0.0011]) for both the worse and fellow eye, respectively. The magnitude of reduction in staining was smaller with lissamine green than with fluorescein, though statistically significant reductions in lissamine green staining were observed for central cornea in the worse eye as well as total conjunctiva, total ocular surface area, inferior cornea and temporal cornea in the fellow eye (Figures 1C and D).
At baseline, reading test results (Ora Calibra Menu, IReST, and Wilkins Rate) varied depending on the type of test but overall suggested a high degree of reading competency in terms of the mean ± SD time to read a passage (42.4 ± 18.4, 54.3 ± 13.6 and 126.0 ± 28.6 seconds, respectively), and the number of words read incorrectly (1.6 ± 2.8, 3.2 ± 5.5, and 16.6 ± 22.0, respectively). After the 6-month treatment period, the time to read a passage decreased modestly when assessed with the Ora Calibra Menu Reading Test (−3.0 ± 14.8 seconds), the IReST Reading Test (-1.2 ± 8.4 seconds) and the Wilkins Rate of Reading Test (-0.7 ± 24.5 seconds), though changes from baseline were not statistically significant (Table 1).
Changes in subject-rated visual function were also assessed following treatment with topical cyclosporine using the OSDI questionnaire. After 6 months of treatment, significant reductions (improvement) were observed in all visual-related questions except for reading (Figure 2). Significant reductions were also observed for the questions related to ocular symptoms and the effect of the environment on dry eye symptoms (Figure 2). Overall, the total OSDI score significantly decreased from 45.1 ± 17.8 at baseline to 31.9 ± 21.3 following 6 months of treatment (-14.9 ± 16.5, p<0.0001). The percentage of subjects with OSDI scores >32 (severe category) was reduced from 76% (38/50) at baseline to 44% (20/45) over the 6-month course of the study.

In the worse eye, there was no significant change in mean ± SD TFBUT from baseline to 6 months (-0.3 ± 1.3 seconds), and there was no significant change in the IBI from baseline to 6 months (-1.2 ± 6.4 seconds). Similarly, there was no significant change from baseline to 6 months in the percentage area of tear deficiency/corneal exposure assessed using the OPI 2.0 (-0.4 ± 1.9).
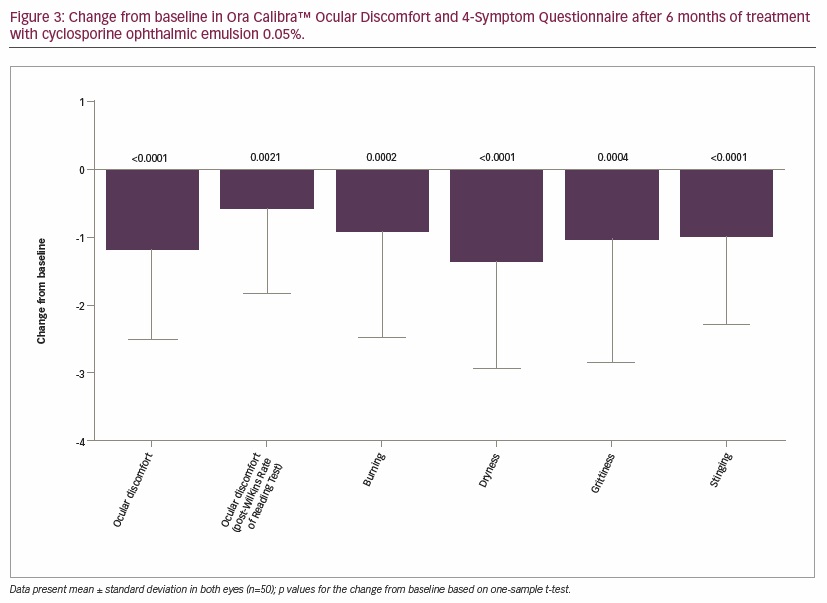
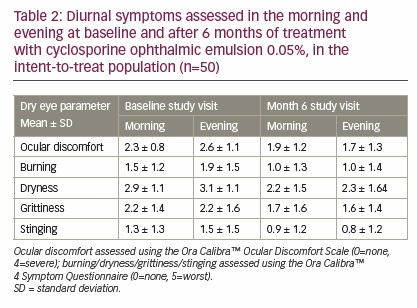
Results of the Ora Calibra Ocular Discomfort Scale demonstrated significant improvements from baseline in both the worse eye (-0.9 ± 1.3, p<0.0001) and the fellow eye (-0.7 ± 1.4, p=0.0012). The Ora Calibra Ocular Discomfort and 4-Symptom Questionnaire also demonstrated significant improvements from baseline in ocular discomfort, as well as burning, dryness, grittiness, and stinging symptoms (Figure 3). A significant improvement was also observed in ocular discomfort after the Wilkins Rate of Reading Test from 3.1 ± 1.0 at baseline to 2.5 ± 1.3 (−0.6 ± 1.2-unit improvement, p=0.0021) after 6 months of treatment. At study visits 2 and 4, diurnal symptoms (ocular discomfort and dry eye symptoms) were assessed in the morning and evening. At baseline, ocular discomfort and burning, dryness, and stinging symptoms were worse in the evening than in the morning, while grittiness showed no change between morning and evening assessments. After 6 months of treatment, ocular discomfort, burning, grittiness, and stinging symptoms all showed improvement in the evening and in the morning, but the magnitude of improvement was generally greater in the evening; some mild worsening in dryness was still observed in the evening compared with the morning (Table 2).
A significant increase in Schirmer’s test score was observed in the worse eye from 7.6 ± 5.5 mm at baseline to 11.4 ± 8.6 mm after 6 months treatment with topical cyclosporine (p=0.0116). Similar results were observed in the fellow eye with an increase in Schirmer’s score from 5.3 ± 3.2 mm at baseline to 9.8 ± 7.3 mm (p<0.0001). Improvement from baseline of ≥10 mm was measured in 16% of worse eyes and fellow eyes, and improvements from baseline of ≥5 mm were measured in 32% of worse eyes and in 38% of fellow eyes after 6 months of treatment.
Safety outcomes
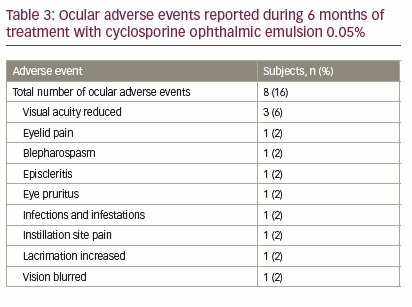
Twenty-two (44%) subjects experienced a total of 36 treatment-emergent AEs, which included eight (16%) subjects who experienced 12 ocular AEs (Table 3). Of the 12 ocular AEs, two were considered possibly related and one was considered definitely related to study drug. Among ocular AEs, reduced visual acuity occurred in three (6%) subjects, and in each case was classified as mild and not related to study drug. Nasopharyngitis was the only non-ocular AE that was reported in ≥5% of subjects (n=3, 6%). Three serious non-ocular AEs were reported during the study (papillary thyroid cancer, peripheral vascular disorder, and carbon monoxide poisoning), none of which were considered to be related to treatment with cyclosporine ophthalmic emulsion 0.05%. The subject diagnosed with papillary thyroid cancer was withdrawn from the study, whereas the other two completed the trial.
Discussion
The last 20 years have seen a rapid rise in the use of computers and hand-held electronic devices, particularly among the younger population, where the use of such devices is typically frequent and prolonged. Excessive and prolonged use of video display terminals can exacerbate signs and symptoms of dry eye.30 Despite the increasing prevalence of dry eye among the young to middle aged (i.e., <50 years of age) and the impact on quality of life,31 dry eye is still often an underdiagnosed and poorly recognized ocular disease in this patient population.
This clinical study was designed to evaluate, in a real-world setting, the efficacy of cyclosporine ophthalmic emulsion 0.05% in dry eye subjects with a history of ocular discomfort and other dry eye symptoms while using video display terminals such as computers or hand-held devices. In this study, significant reductions in corneal and conjunctival staining were observed, consistent with prior studies of topical cyclosporine.20 In addition, significant improvements in visual function OSDI scores (blurry vision, poor vision, driving at night, working with computer or bank machine, and watching television) were also observed after 6 months of treatment. Similar findings have been reported previously in a single-center, 6-month, open-label study in 40 dry eye subjects, who were significantly older (mean age 59 years, range 40–78) than the population in the present study (mean age 46 years, range 25–55).32
In addition to the reduction in overall staining, a significant reduction in fluorescein staining was also observed in the central corneal region, which may contribute to improvement in subject-rated visual function.33,34 Significant reductions in conjunctival staining have also been reported in previously published studies investigating the use of topical cyclosporine in patients with dry eye.20,35,36 Similarly, as reported elsewhere,20,32,37,38 the use of cyclosporine has been associated with a significant increase in tear production assessed by Schirmer’s test scores. In the present study, nearly half of the subjects (47.6%) originally categorized with severe disease (OSDI score >32) at baseline improved by at least one category in dry eye disease severity after 6 months of treatment. Perry et al. previously reported similar findings where the use of topical cyclosporine led to improvements in OSDI scores in 158 patients with mild, moderate, or severe dry eye disease.36 Cyclosporine ophthalmic emulsion 0.05% was well tolerated in the study, consistent with its established safety profile.20,21,32,35
Visual functioning assessed using the Ora Calibra Menu Reading Test, IReST Reading Test, and the Wilkins Rate of Reading Test showed improvements following treatment with topical cyclosporine, although differences in time to read passages and the number of words read incorrectly did not reach statistical significance. OSDI visual function scores did show significant improvement (reduction) from baseline. No studies have been conducted correlating reading test results and subject-rated visual function. Our study demonstrates improvement in both assessments, suggesting subjects experienced benefit in vision-related outcomes following 6 months of treatment with topical cyclosporine.
Dry eye disease is often associated with symptoms that vary throughout the day, often leading to difficulties in evening visual activities such as reading, television or computer use. Over the course of a day, the combination of environmental exposure and visual tasking activities may have a cumulative effect on the ocular surface resulting in a measurable pattern of progressive increase in signs and symptoms.5,39 Treatment with topical cyclosporine demonstrated significant reduction in diurnal symptoms assessed in this study. After 6 months, subjects demonstrated improvements in ocular discomfort, burning, grittiness, and stinging symptoms in both the evening and the morning, with even more robust reductions seen in evening symptoms relative to baseline.
Treatment with cyclosporine ophthalmic emulsion resulted in significant symptom relief. Significant improvements in symptoms measured using the Ora Calibra Ocular Discomfort Scale and Ora Calibra Ocular Discomfort and 4-Symptom Questionnaire were observed following 6 months of treatment. In addition, ocular discomfort scores collected following Wilkins Rate of Reading Tests were significantly reduced in this study. In the pivotal phase III trials involving 877 patients, significant improvements from baseline were observed in dryness, sandy/gritty feeling, burning, and stinging symptoms following the use of cyclosporine.20 Using the Ora Calibra Ocular Discomfort and 4-Symptom Questionnaire, significant improvements in ocular discomfort and dry eye symptoms have also been reported following cyclosporine instillation in the single-center, 6-month, open-label study.32
The lack of a comparator or control group could potentially be a study limitation. However, within-subject comparisons often provide the most reliable data for conditions such as dry eye disease, which has a highly variable presentation of signs and symptoms due to differences in exogenous factors that impact each subject. Variations in environmental conditions and disease phenotype across subjects can create significant disparities in the spectrum of individual dry eye pathophysiology. The lack of a placebo/control group does preclude attributing any of the AEs reported in this study to the effects of cyclosporine ophthalmic emulsion 0.05%. In addition, the reading tests used were likely too short to show a change in reading speed in patients with normal vision and dry eye disease. Although there was improvement in corneal staining which translated to significant subjective improvement in visual function, this did not translate into faster reading.
Conclusion
Although dry eye disease is often considered to affect the elderly,40 a growing body of literature has confirmed the rising prevalence of dry eye in the younger adult population. This study demonstrates the efficacy and safety of cyclosporine ophthalmic emulsion in treating a younger dry eye population who experience ocular discomfort when engaging in visual tasking. Following 6 months of treatment with cyclosporine ophthalmic emulsion, significant improvements were observed in ocular signs, symptoms, and subject-rated visual function.







