As our life expectancy, along with our sedentary lifestyles increase, the prevalence of diabetes mellitus increases as well. With increasing prevalence and duration of diabetes, the incidence and prevalence of diabetic retinopathy grows as well. Diabetic macular oedema (DMO) is a frequent cause of vision loss in people with diabetes and is one of the leading causes of vision loss among people with diabetic retinopathy. DMO is the retinal thickening from accumulation of intracellular or extracellular fluid caused by hyperpermeability of the inner endothelial blood-retinal barrier. The main leakage site are abnormally permeable microaneurysms, intra-retinal microvascular abnormalities (IRMA) and damaged retinal capillaries. Treatment of DMO can be complicated due to varying patient response to treatment approaches. The most novel and effective treatment presently is combination therapies with anti-vascular endothelial growth factor (VEGF) injections and corticosteroids.1
Epidemiology
Diabetic retinopathy affects approximately 93 million people worldwide, and is the leading cause of vision loss in adults aged 20–74 years.2 Out of those 93 million, 21 million have DMO. The overall prevalence of DMO is 6.81% for DMO in people with diabetes worldwide, accounting for 12% of new cases of blindness, annually. Studies have shown that 24% of eyes with DMO will lose at least three lines of vision within 3 years.3 The Diabetes Control and Complications Trial (DCCT) reported that 27% of patients with type 1 diabetes develop DMO within 9 years of onset.4 Further, from 1980–2011, the prevalence of diagnosed diabetes increased 176% in the US.5
Pathophysiology
Early detection and intervention in DMO is key to reducing the risk of permanent vision loss. Therefore, understanding the pathophysiology of DMO is vital to progression in treating DMO. DMO causative factors include both inflammatory and ischaemic mechanisms. DMO is associated with hyperglycaemia as well as oxidative stress; endothelial and pericyte cells along with the associated tight junctions in the blood-retinal barriers degrade, causing blockage of capillary perfusion to retinal and neural cells. The body’s response is the release of cytokines, chemokines and other mediators such as VEFG. VEFG also increases leukocyte adhesion to capillaries, which contributes to endothelial and neuronal apoptosis. Other inflammatory mediators implicated in diabetic retinopathy and DMO development are chemokines like C-C motif chemokine ligand 2 (CCL2), integrins transmembrane receptors, insulin-like growth factor (IGF-1), platelet-derived growth factor, and basic fibroblast growth factor.
The diagnosis of DMO takes into account location of retinal thickening relative to the fovea, both clinically and by optical coherence tomography (OCT) micro-anatomy. DMO is classified as centre-involving or non-centre-involving, and OCT-based classifications have been proposed that better allows extension in characterisation of volume and morphologic features.6 However, it is important to remember that protocol A (Diabetic Retinopathy Clinical Research network [DRCR.net]) concluded that there is only “a modest correlation between OCT-measured centre point thickness and visual acuity, and modest correlation of changes in retinal thickening and visual acuity following focal laser treatment for DMO”.7 Even though OCT parameters do not reliably reflect the level of visual acuity in patients, it allows us to more precisely define the different patterns, extent of progression and response to treatment in a reliable, precise and reproducible way so that we may judge and compare different therapeutic strategies.7
Treatment evolution
One of the first lines of defence when treating DMO has always been to reduce systemic risk factors, such as elevated blood glucose levels, lipids, and blood pressure. DRCR.net has concluded that “results suggest that optimising glycaemic control remains a substantive challenge requiring more extensive interventional paradigms.”8 Other treatments include focal and scatter laser treatment, vitrectomy, corticosteroids, and finally anti-VEFG therapy. The history of these treatments, as well as how prevailing they are, have much to do with the short- and long-term side effects and outcomes in vision. The introduction and rapid adoption of intravitreal pharmacologic agents, particularly drugs that block VEGF and corticosteroids, have changed the goal of diabetic retinopathy treatment from stabilisation to improvement of vision.
Focal/Grid laser comes with the risk of scarring the retina. Before the discovery of anti-VEFG therapy, photocoagulation was used singularly when treating DMO, and has been the mainstream method of DMO treatment for the past 25 years.9 Photocoagulation is used to treat microaneurysms and focal areas of retinal thickening.10
Intravitreal therapy
Corticosteroids are (steroids) received via intravitreal injections, or by sustained released implant. They can improve vision by interfering with the inflammation and ischaemic mechanisms that damage the retinal-blood barrier and may also inhibit neovascularisation.11,12 However, steroid therapy is known for causing complications such as cataracts, elevated intraocular pressure (IOP; a major risk factor of glaucoma) and endophthalmitis.13,14 Long-lasting sustained-release corticosteroids (e.g., Iluvien® fluocinolone acetonide [Alimera Sciences, Inc., GA, US] and Ozurdex® dexamethasone [Allergan, Dublin, Republic of Ireland]) allows providers to treat with minimal intervention, and allows for more control since patient visits can be spread out over time. With recurrent injections, follow-ups are crucial to the treatment of DMO.
The MEAD study examined the safety and efficacy of dexamethasone intravitreal implant in DMO.15 Patients were randomised 1:1:1 to dexamethasone implant 0.7 mg, dexamethasone implant 0.35 mg, or sham, and were followed for 3 years. The main outcome measurement was improvement of ≥15 logMAR letters from baseline to the end of the study. The mean reduction in central retinal thickness from baseline was greater in the groups receiving dexamethasone implants with 0.7 mg and 0.35 mg versus sham. The MEAD study reported clinically significantly increased IOP in patients treated with dexamethasone: 36% (0.7 mg group), 34% (0.35 mg group) and 18% (sham). The BEVORDEX study compared bevacizumab with dexamethasone implant, and concluded that despite similar reports in improved logMAR letters in both treatment groups (40% bevacizumab, 41% dexamethasone), none of the 42 eyes receiving bevacizumab lost 10 letters or more, while 11% of eyes on dexamethasone did, mostly due to cataract.16 The FAME trial (fluocinolone insert) proved that DMO patients of >3 years duration responded better to the insert than did those with non-chronic DMO.17 Some proposed that chronic DMO is chemokine-driven while non-chronic DMO is VEGF-driven.17
Anti-vascular endothelial growth factor therapy
Over the past few years intravitreal injections of anti-VEGF agents have progressively replaced focal laser photocoagulation for the primary treatment of centre-involving DMO. Anti-VEFG decreases overexpression of protein endothelial growth factor, thus reducing vascular permeability and oedema of the retina.18 The three key anti-VEFG therapies are ranibizumab (Lucentis®, Genentech USA, Inc., CA, US), aflibercept (Eylea®, Regeneron Pharmaceuticals, Inc., NY, US) and bevacizumab (Avastin®, Genentech USA, Inc., CA, US). Aflibercept is an intermediate-sized fusion protein that binds to all isomers of the VEGF-A family. Aflibercept differs from ranibizumab and bevacizumab in that it binds VEGF-A more tightly than its native receptors and it also binds other VEGF family members, such as placental growth factor.19 Major anti-VEGF treatment trials for DMO have used laser as well as a rescue therapy. The DRCR.net, through Protocol I, combined ranibizumab with immediate or deferred (6 months) laser;20 Protocol T allowed focal/grid laser photocoagulation for persistent DMO at 24 weeks;21 RISE and RIDE allowed laser after 3 months;22 and VIVID/VISTA trials focus laser photocoagulation (with sham intraocular injections) compared to intravitreal aflibercept every 4 or 8 weeks after initial monthly dosage.23
RISE and RIDE were two, phase III clinical trials, with sham-controlled groups and ranibizumab injections over a 24–36-month period. Both trials analysed sham versus 0.3 mg ranibizumab versus 0.5 mg ranibizumab monthly monotherapy.22 At 36 months, a gain of >15 letters was experienced by 36.8–51.2% of ranibizumab-treated patients, with similar reductions of central foveal thickness (CFT); 38.9–39.2% of eyes demonstrated a two-step regression in diabetic retinopathy severity, and 13.2–15.0% demonstrated a three-step regression.22 Further, ranibizumab patients underwent significantly fewer macular laser procedures. Overall, there was minimal systemic cardiovascular complications associated with inhibition of endothelial growth factor. RISE and RIDE trials proved ranibizumab to be a safe and effective drug to use as a monthly monotherapy for sustainable treatment of DMO.22 An open-label extension phase (14 months) revealed the visual gains to be maintained with a decreased frequency of treatment.24
Bevacizumab is a very commonly used anti-VEGF agent due to its low cost. In the BOLT trial, patients were randomised to injections versus laser. At 1 year the bevacizumab group’s (n=42) visual gains were 8 letters whereas the laser group (n=38) lost 0.5 letters.25
Diabetic Retinopathy Clinical Research Network
In 2010, Protocol I in the DRCR.net study demonstrated the dominance and efficacy of ranibizumab with immediate or deferred laser instead of laser alone when treating centre-involving DMO in 1 year.20 In 2012, Protocol I made the same conclusions at the 3-year mark26 and then again in 2016 at the 5-year mark.27
In April of 2017, Protocol T (DRCR.net) showed comparative data for the three anti-VEFG drugs available.21 For eyes with baseline visual acuities of 20/32–20/40, visual gain was similar (+8 letters). But for eyes with baseline visual acuity of 20/50 or worse, aflibercept (+18.9 letters) produced greater gains in visual acuity than ranibizumab (+14.2 letters) and bevacizumab (+11.8 letters). Aflibercept-treated patients also had greater macular thinning (-169 µm) than either ranibizumab (-147 µm) or bevacizumab (-101 µm).21 The 2-year results from comparative effectiveness of the three drugs showed that superiority of aflibercept over ranibizumab noted at 1 year, was no longer significant. Another study has shown that increasing the dosage of ranibizumab may have additional benefits to vision, so if using ranibizumab is the only option it is important to keep this in mind.28,29
Protocols I and T used monthly injections for 4 months or until OCT was dry before switching to monthly pro re nata (PRN) protocols based on strict retreatment criteria. PRN treatment regimens reduce the number of injections, but not the number of office visits. The RETAIN trial compared treat and extend (T&E) + laser, T&E and PRN ranibizumab regimens over 24 months in patients with DMO.30 Patients in all groups were treated monthly until dry. Visual acuity improvement in all groups was similar (T&E + laser, +5.9 letters; T&E, +6.1 letters; PRN ranibizumab, +6.2 letters), and the mean number of injections were 12.4, 12.8 and 10.7, respectively, but patients treated with T&E required 46% fewer office visits. Over 70% of patients had treatment intervals extended to at least 2 months. These results are encouraging and although a multi-centre, randomised trial comparing monthly therapy with T&E would be useful, this will not deter physicians from employing a T&E approach when treating DMO.
DMO is a complex disease, and with the introduction of potent therapeutic agents we have seen dramatic results over the past decade. Although anti-VEGF therapies continue to evolve, the future holds tremendous promise for new novel therapies. Careful patient selection is key to optimising treatment outcomes as new pharmacologic drugs are added to our treatment armamentarium.
Case report
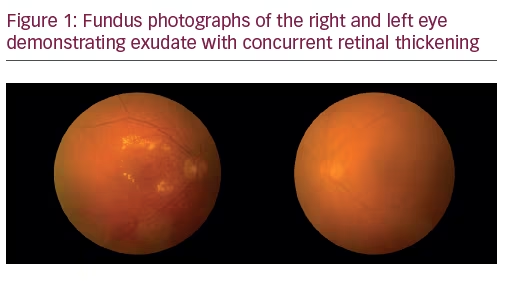
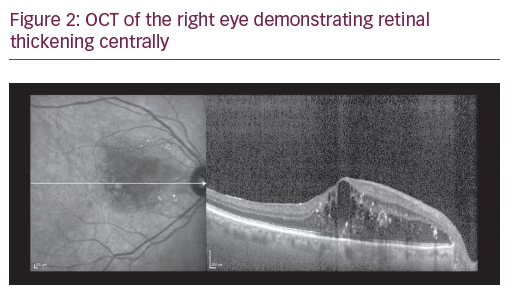
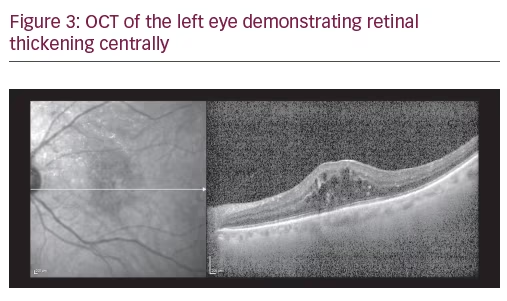
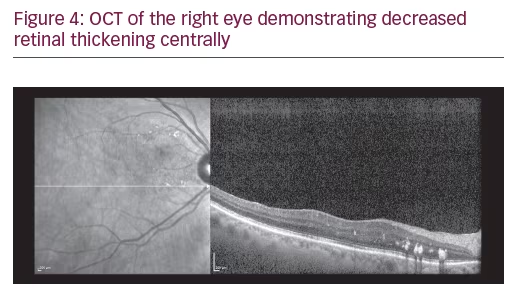
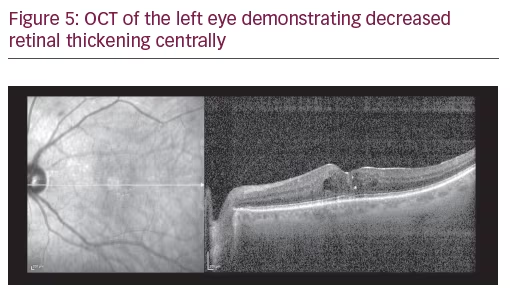
A 75-year-old Caucasian female presented for ongoing treatment of DMO. After recent treatments with intravitreal bevacizumab, visual acuity was 20/400 OD and 20/200 OS. Funduscopic examination revealed non-proliferative diabetic retinopathy with DMO OU (Figure 1). OCT revealed persistent thickening OU (Figures 2 and 3). Aflibercept 2 mg was injected intravitreally OU. Upon follow-up, 2 months later, visual acuity had improved to 20/60-2 OD and 20/70+1 OS. Funduscopic examination revealed reduced thickening, with the OCT confirming the clinical findings (Figures 4 and 5). The patient received a repeat intravitreally injection of aflibercept to the left eye, with observation of the right eye.







