Diabetic macular oedema (DMO), a pathological condition characterised by the breakdown of the blood–retina barrier and a consequent increase in vascular permeability, is one of the most challenging conditions faced by ophthalmologists today, and its prevalence is rising. The global prevalence of diabetic retinopathy among individuals with diabetes is around 35 %, with DMO present in 6.8 %.1 Despite the success of agents targeted against vascular endothelial growth factor (VEGF) including ranibizumab,2,3 bevacizumab3 and aflibercept,4 significant unmet needs remain in the treatment of chronic DMO; around a third or more of patients are considered insufficiently responsive to anti-VEGF therapy.5 Recent research has elucidated the multi-factorial pathogenesis of DMO and focused on patients with advanced disease, who exhibit numerous inflammatory changes in the eye.6–9 At this stage, VEGF is no longer primarily responsible for the biochemical and physiological changes in the eyes and anti-VEGF agents are consequently inappropriate as DMO is a multi-factorial disease.10,11
These findings in DMO pathogenesis have led to a resurgence of interest in corticosteroids, which suppress multiple pathways of inflammation and reduce damage to the blood–retina barrier.12 Intravitreal steroids, particularly in sustained-release implants, offer an alternative therapeutic strategy, providing localised delivery of the corticosteroid to maximise its anti-inflammatory, angiostatic and anti-permeability effects, as well as minimising the risks of systemic toxicity.13 Treatment options include: a dexamethasone implant (Ozurdex®), the fluocinolone acetonide (FAc) intravitreal implant and intravitreal triamcinolone acetonide (TA) injection (the latter is not approved for treating DMO in Europe). ILUVIEN® (fluocinolone acetonide) delivers 0.2 μg/day of FAc and is the only therapy approved for the treatment of chronic DMO.14 It received marketing authorisation in a number of European countries as well being approved by the US Food and Drug Administration for the treatment of DMO.15,16 This article aims to assess some recently published case series reports of the early use of FAc intravitreal implants in a real-world setting at treatment centres in Germany and the UK, as well as assessing their impact on future chronic DMO treatment practice.
Clinical Background of the Fluocinolone
Acetonide Intravitreal Implant
The FAc intravitreal implant is a non-bioerodible micro-implantable cylindrical tube (3.5 mm × 0.37 mm) made from polyimide and loaded with 190 μg of FAc17 that is inserted into the vitreous cavity using a 25-gauge injector, which creates a self-sealing wound. The implant releases 0.2 μg/day of FAc for up to 36 months.17 The pivotal Fluocinolone Acetonide for Macular Edema (FAME) clinical trials were two large prospective, randomised, controlled studies that followed 953 eyes randomised to receive 0.2 μg/day of FAc (low dose; the licensed dose) or 0.5 μg/day of FAc (high dose) implants or sham. After 3 years, substantial improvements were seen in mean visual acuity in 768 patients treated
with FAc (high or low dose) compared with 185 who received sham treatment.17,18 A preplanned subgroup analysis, which underpinned the licensed indication for FAc intravitreal implants in Europe, assessed outcomes in patients with chronic DMO. The percentage of chronic DMO patients who gained 15 letters of best-corrected visual acuity (BCVA) or more from baseline at month 36 was 13.4 % in the sham group and 34.0 % in the 0.2 μg/ day FAc intravitreal implant group (p<0.001).19 Side effects were manageable and included cataract formation and increased intraocular pressure (IOP). The results showed that patients with chronic DMO who tend to respond poorly to many treatments, including focal/ grid laser photocoagulation, responded well to the administration of a FAc intravitreal implant.
Real-world Data
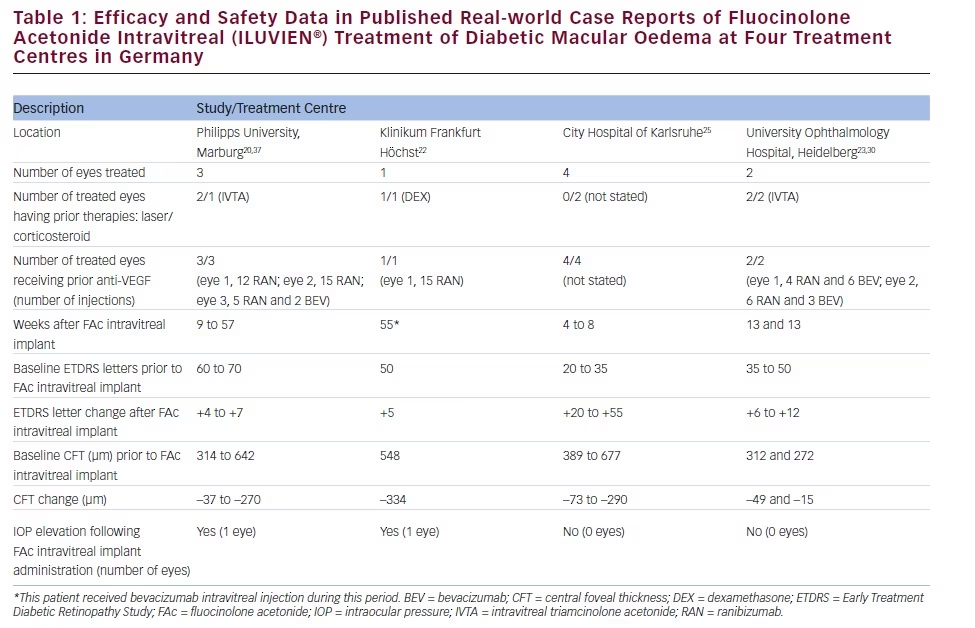
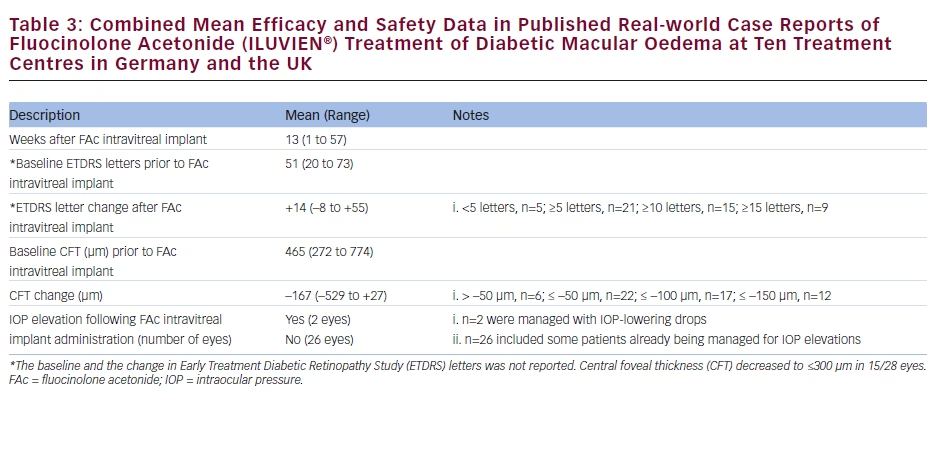
There is a growing amount of real-world clinical data on the use of FAc intravitreal implants to support their use in chronic DMO.13 Recently, several case reports have been published, detailing early experiences with FAc intravitreal implants in real-world clinical use at ten treatment centres in Germany and the UK (see Tables 1–3).20–26 These reports include 28 cases (eyes) with a mean follow-up of 13 weeks (range 1 to 57 weeks). To enable comparison and consistency, the units reported from different case series have been standardised for this review (see Table 4 for conversion scales; visual acuity measurements were converted to number of Early Treatment Diabetic Retinopathy Study [ETDRS] letters.) Patients had received prior treatment including corticosteroid (16/28, 57.1 %), laser (16/28, 57.1 %) or anti-VEGF (26/28, 92.9 %). Patients received a total of 148 prior intravitreal injections of anti-VEGF (119 ranibizumab and 29 bevacizumab injections, respectively), an average of 5.3 intravitreal injections per eye prior to the FAc intravitreal implant. This is reflective of current clinical practice and standard of care. Prior to FAc intravitreal injection, all patients had a baseline central foveal thickness (CFT) ≥272 μm and a baseline ETDRS letter score between 20 and 73. These figures are similar with the inclusion criteria for the FAME studies (CFT ≥250 μm and BCVA between 19 and 68 letters [20/400 to 20/50 or 20 to 65 ETDRS letters]).17
The FAc intravitreal implant exerted rapid effects, sometimes showing gains in visual acuity and reductions in CFT within a week26 and sustained for the duration of the follow-up. Data are now available for cases with 1 year or more follow-up.20,22 In the current cases, there was a mean follow-up of 13 weeks after implantation (range 1–57 weeks); there was a general improvement in visual acuity reported by the majority of the centres, with an average increase of 14 letters. Just over 50 % achieved ≥10 letters and 32.1 % achieved ≥15 letters from baseline, respectively. At all treatment centres, there was a notable decrease in CFT from a mean 465 μm to 298 μm (–167 μm) and CFT decreased to ≤300 μm in 15/28 cases (53.6 %).
The use of FAc intravitreal implants is associated with accelerated development of cataract, and elevated IOP, both of which are known side effects of corticosteroid therapy.27 In the case series, IOP rises occurred in 2/28 (7.1 %) compared with 38.4 % in the FAME clinical trials.17 Both cases were managed with IOP-lowering drops.
accounted for 45.4 % (n=54) of reported ranibizumab injections, with an average of 5.4 injections per eye in Germany versus 3.6 in the UK (when bevacizumab is included, the figures are 6.5 in Germany versus 4.6 in the UK). The real-world efficacy of the FAc intravitreal implants in the case series was similar to that seen in the FAME study, with a mean gain in the number of ETDRS letters of +14 (–8 to +55) and a decrease in CFT of 167 μm (range –529 to +27; see Table 3). This is an encouraging finding and could be due to the pharmacokinetic properties of the FAc intravitreal implant in providing low dose and sustained released of FAc for up to 36 months after a single injection. As well as the efficacy and safety demonstrated in the case series, FAc intravitreal implants have been associated with improvements in visual quality of life and the ability to perform daily routines as was reported in a case of a 62-year-old man in Germany.22 In another reported case, treatment improved the patient’s confidence to return to work and restart his previous job.20 Improvements in visual acuity to 0.5 or 20/40 is an important functional outcome as this is the minimum requirement for maintaining a driving licence in Europe.31 In addition to the case series described, a recent report describes initial post-marketing experience in the Birmingham and Midlands Eye Centre, Birmingham, UK.32 Over 9 months, the implant has been used to treat DMO in 23 eyes of 18 patients, all of whom had failed prior therapy and had chronic, advanced macular changes associated with DMO duration of 2 to 7 years. After follow-up ranging from 1 week to 9 months, the mean subfoveal thickness decreased from 513 μm pre-implantation to 376 μm post-implantation. Significant DMO was reported in six eyes following FAc intravitreal implant injection but these cases had a relatively short follow-up (5 days to 2 months). Improvements in visual acuity were noted in most eyes, with some gaining up to 4 Snellen lines of acuity. Only thrree eyes had no improvement on optical coherence tomography (OCT) or in visual acuity. These unresponsive patients had also failed prior treatment with laser, anti-VEGF injections, and even intravitreal triamcinolone. Patient satisfaction was also high, a result of the reduced visit burden. Instead of monthly treatment visits, patients who received the FAc intravitreal implant were scheduled for follow-up visits every 3 months after an initial follow-up at 1 to 2 weeks and then 6 to 8 weeks post-implantation. Improvements were observed at an early stage following implantation: within 2 weeks, with further benefit reported at 6 weeks. The study author considered that if no benefit is seen by 6 weeks, improvement is unlikely to occur with longer followup. To date, the only post-implantation adverse event was elevated IOP in one eye (28 mmHg), which was controlled with topical medication.32 The study author also concluded that the indications for FAc intravitreal implants should be expanded to include eyes with better macular structural integrity and visual prognosis as well as for patients who had failed only laser therapy and expressed a desire to avoid monthly anti-VEGF injections. The Importance of Early Treatment in Diabetic Clinical evidence shows that earlier treatment reduces visual loss in diabetic retinopathy. It is important to identify corticosteroid responders and anti-VEGF non-responders early in the disease course and initiate suitable treatment for each patient. Earlier therapy results in improved functional outcomes i.e. gaining 20/40 vision, which is satisfactory for most occupations and lifestyles, enabling ability to drive, ability to work and having the confidence to return to work. Treating later in the disease course will not restore visual function to this level and could thus prevent individual patients from being able to work or function normally in other areas of their lives.33 Some physicians adopt a ‘watch and wait’ approach in DMO, but this is counterproductive and may lead to unnecessary and harmful treatment delays; if the patient has not sufficiently responded to a prior course of anti-VEGF therapy, then treatment with FAc intravitreal implants should be considered to acheive optimal visual outcomes.34 The definition of an insufficient VEGF responder, however, has not been conclusively established. The number of anti-VEGF treatments that should be given before a patient is considered an insufficient responder remains to be determined. Some experts suggest three to six treatments are sufficient. At the author’s centre in Sheffield, patients are given at least three to four monthly anti-VEGF injections and are then reviewed 14 days later to assess for at least a 130 μm reduction in macular thickness with a 5 to 7 letter gain in vision. A DMO case not achieving these outcomes would be deemed insufficiently responsive to anti-VEGF and a switch to FAc intravitreal implant would be discussed with the patient. Guidelines on the Use of the Fluocinolone The UK National Institute for Health and Care Excellence (NICE) recommends the FAc intravitreal implant as an option for treating chronic DMO that is insufficiently responsive to available therapies if the implant is to be used in an eye with an intraocular (pseudophakic) lens.35 The implant is available on the UK National Health Service (NHS) through a patient access scheme. However, the implant has been successfully used in phakic eyes,17–19,36 and there is therefore the potential to widen its application. Such a restriction to only pseudophakic eyes does not apply in the rest of Europe or the US. Summary and Concluding Remarks Real-world data on the use of the FAc intravitreal implant has shown efficacy equivalent to that seen in the FAME clinical trials, and, in the limited number of reported cases in the public domain, the number of patients experiencing an elevation in IOP may be fewer than reported in the FAME studies. This suggests that FAc intravitreal implants should be considered in DMO patients that do not respond optimally to anti-VEGF and/or laser treatments. However, it must be noted that the real-world data set is, as yet, small and this is a limitation that should be addressed by assessing larger patient populations. The use of FAc intravitreal implants has been associated with increased patient satisfaction and a lower treatment burden with FAc versus others using frequent intravitreal injections. If lower incidence of IOP elevation, as seen in the real-word case series, is sustained in other real-world studies, this will increase confidence and further support the use of FAc intravitreal implants in DMO.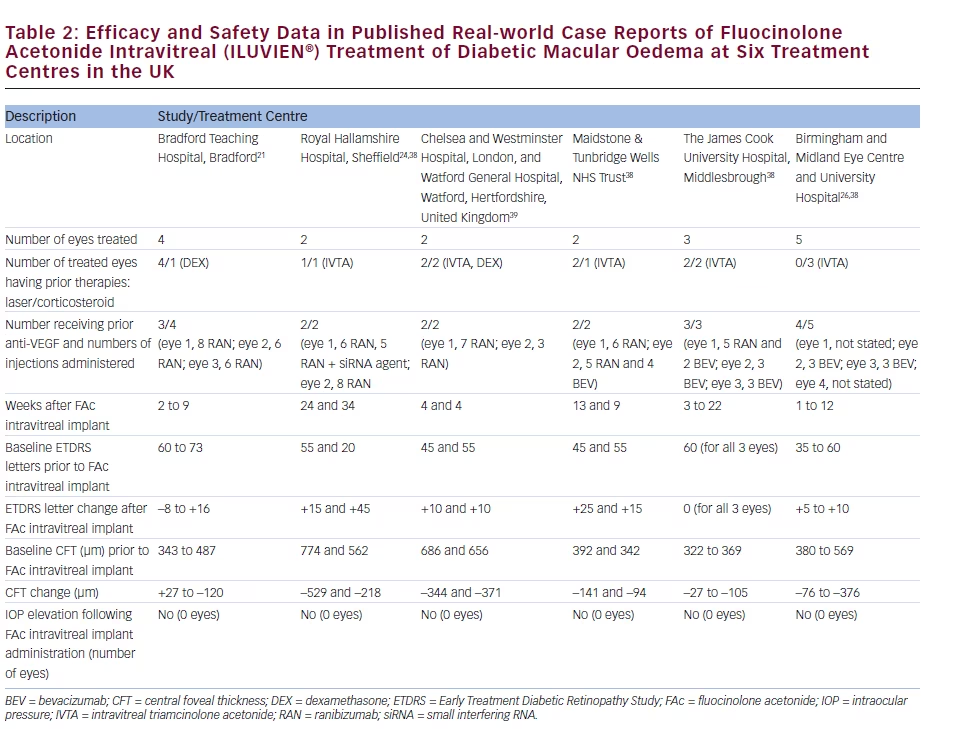
Macular Oedema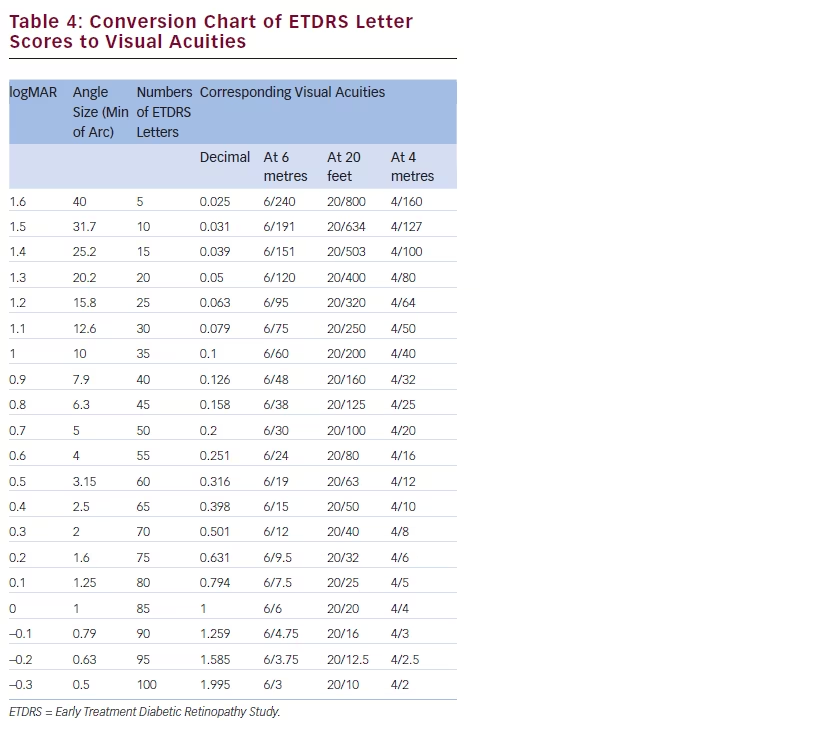
Acetonide Intravitreal Implant







