Varicella zoster virus is a double-stranded DNA herpesvirus that resides dormant in the sensory nerve ganglia after primary infection of varicella/chicken pox. While primary infection can be mild, on reactivation, the virus typically causes a painful vesicular rash called herpes zoster (HZ), that affects approximately 1 million people per year in the USA.1 The incidence of HZ has quadrupled in the past 60 years and continues to increase worldwide.2 Although it is generally more common with age, the incidence in younger patients is rising more quickly, and the average age of initial presentation of HZ is decreasing, with reports citing an average age of 56–59 years.3–5 It has been argued that this change may be related to the increase in childhood varicella vaccinations, but the incidence of HZ has been increasing in countries where widespread vaccination is not given and has also been stable in patients over age 65, so other factors are likely involved.4–7 The sequelae of HZ outside of ocular disease include chronic pain secondary to post-herpetic neuralgia (PHN), and potentially lethal systemic complications, including stroke.8,9 Around 10–20% of HZ involves the ophthalmic branch of the trigeminal nerve and is called herpes zoster ophthalmicus (HZO).10–12
General presentation and management of herpes zoster ophthalmicus
HZ typically results in a painful, unilateral, dermatomal vesicular rash. Ocular involvement in HZO is common, especially when the Hutchinson sign (lesions on the nose) is observed: in one case series of 259 patients with HZO, 66.0% had ocular involvement.13 The most frequent ocular manifestations of HZO in those patients were conjunctivitis (56.8%) and anterior uveitis (17.8%). Keratitis is also a common presentation, with an incidence that varies by subtype, including stromal (6.6%), epithelial (5.0%) and endothelial (1.2%).13 Anterior uveitis can be an infectious manifestation in both early and late forms.14 HZO can also result in scleritis, trabeculitis, optic neuritis, retinal vasculitis, giant cell arteritis, retinopathy and cranial nerve defects.10,15 After acute presentation, HZO can progress into chronic or recurrent disease. Chronic HZO occurs in 23.0% of patients, and recurrence rates increase with time from 8.0% of patients at 1 year to 25.0% of patients at 5 years after the original acute HZO onset.16 The early manifestations of herpes zoster keratitis (HZK) are related to the active virus, and present as dendriform epithelial keratitis. Later forms of HZK are more related to an inflammatory immune response, and can cause stromal keratitis or endotheliitis. After appropriate management of acute or recurrent corneal disease, long-term damage from HZK can result in neurotrophic keratitis (NK) and corneal scarring.3,8
The management of HZ involves prevention with vaccination. Vaccination is recommended by the Centers for Disease Control and Prevention (CDC) for all immunocompetent patients over the age of 50 years. There are two vaccines available worldwide: a single-dose zoster vaccine live (ZVL) that is 51% effective (Zostavax®; Merck & Co. Inc., West Point, PA, USA) and a two-dose recombinant zoster vaccine (RZV) that is 96.0% effective (Shingrix®; GlaxoSmithKline, Rixensart, Belgium).17,18 In addition to being more effective than the ZVL, the RZV has fewer contraindications, although it does require two doses and causes more acute side effects. The most common side effects of vaccination are local injection-site reactions or mild systemic reactions, but rare ophthalmologic reactivation of HZ has been reported, sometimes with severe consequences, including corneal perforation.16,19,20 The RZV is approved by the US Food and Drugs Administration for adults aged 50 years and older, and is preferred over the ZVL, which is no longer available in the USA since 2020. The RZV is recommended by the CDC for persons with a history of zoster or past vaccination with ZVL.21
Oral antiviral treatment options for HZ include 1 g valacyclovir three times daily, 800 mg acyclovir five times daily or 500 mg famciclovir three times daily, with initiation of therapy within 72 hours of symptom onset for a duration of 7–10 days.22–24
Early and late manifestations of herpes zoster keratitis
Early HZK can present as dendriform epithelial keratitis, and is related to active virus and should consequently be treated with antiviral medications.3,8 Treatment is often with the same oral antiviral medications as for HZ, typically acyclovir or valacyclovir, but in refractory cases topical ganciclovir 0.15% gel five times daily has been reported to aid management.25 Epithelial disease can occur later as mucous plaque keratitis or delayed pseudodendrites and, in addition to oral antivirals for these patients, the mucous plaques can be debrided and topical acetylcysteine 10.0% drops can be added.8,26
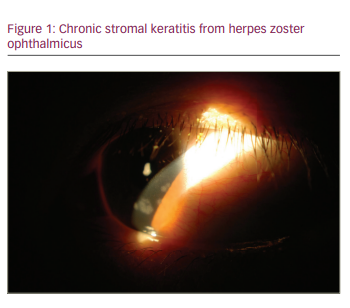
The treatment of inflammatory complications of HZK, such as stromal or endothelial keratitis, centres on topical steroids. The most commonly used regimen is prednisolone acetate 1% four times daily, but surveys of current treatment among cornea providers show that a range of topical low- and high-potency steroids are used at different dosing frequencies ranging from daily to greater than five times daily.27 Stromal or nummular keratitis (Figure 1) can lead to complications such as corneal scarring, neovascularization, ulceration or lipid keratopathy that may require surgical interventions.3,8 Endotheliitis, or disciform keratitis, likewise responds well to topical steroids but, if significant endothelial cell loss occurs, surgical intervention such as endothelial keratoplasty (EK) or penetrating keratoplasty (PK) may need to be considered, depending on the degree of stromal damage.26 PK does carry the risk of increased complications over EK, including secondary glaucoma and ocular surface problems due to NK that may benefit from tarsorrhaphy.28 Iritis can occur as part of early or late HZO.3,8
Complications from herpes zoster keratitis
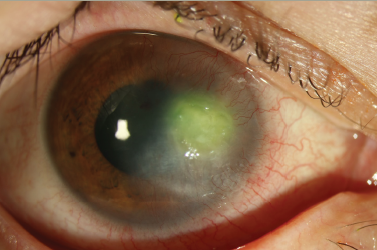
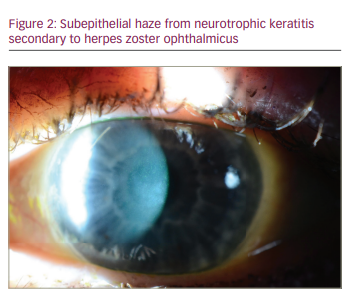
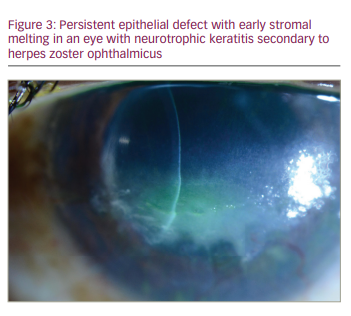
NK is a complication that can also develop from damage to the corneal nerves from HZO (Figure 2), and this can lead to secondary complications, such as persistent epithelial defects (Figure 3), microbial superinfection and perforation.8.29 NK is more common in older patients, and it can be managed with lubrication, punctal plugs, tarsorrhaphy or scleral lenses.24 Recombinant human nerve growth factor and corneal neurotization are newer modalities to treat and address NK.29 Corneal perforations, if smaller than 1.5 mm, can be managed with cyanoacrylate or fibrin glue and ocular antihypertensives. Amniotic membrane or conjunctival grafts and flaps can also help but, in severe cases, a PK may be required.29
Secondary corneal scarring and astigmatism can be managed with contact lenses and surgical options, including phototherapeutic keratectomy (PTK) and PK.30 Rigid gas permeable lenses can be used if there is normal or mildly decreased corneal sensation but, in cases of more severe neurotrophic keratopathy, scleral contact lenses are a better option.8 Corneal surgical procedures carry the risk of persistent epithelial defects and subsequent stromal melting, as surgery can worsen the pre-existing neurotrophic state, and outcomes are best when active inflammation, intraocular pressure and NK are optimized pre-operatively with artificial tears, punctal plugs, cyclosporine and topical or oral antivirals.26 For stromal scarring, PTK with mitomycin 0.02%, or deep anterior lamellar keratoplasty can be considered. For severe endothelial dysfunction, EK should be considered, and for combined stromal scarring and endothelial dysfunction, PK can be considered. In either scenario, any active disease should be quiescent for 3 months prior to surgery, and serious consideration should be given to starting treatment dosing of oral antivirals several weeks prior to surgery, continuing through the initial post-operative period. Subsequently, oral antiviral prophylaxis should be maintained.8 Keratoprostheses have also been used, but these eyes tend to have a worse outcome than in cases with herpes simplex virus (HSV).31 Conjunctival grafts or flaps can be used for peripheral ulcers or persistent epithelial defects from NK, and exposure can be managed surgically with tarsorrhaphy or eyelid weights.8
Chronic and recurrent herpes zoster keratitis
Management of chronic or recurrent disease can be challenging, and topical corticosteroids have been the mainstay of treatment. However, the concept of recurrence or chronicity of HZK prompts discussion of prophylaxis, as there is evidence that, during clinically quiescent times, subclinical viral transcription and translation may occur when an intact cell-mediated response is disrupted.32–34 Unfortunately, there is no consensus on exact dosing, frequency, duration or even effectiveness of treatment. Long-term prophylaxis with oral antivirals is commonly used by cornea specialists, with different dosing including 1 g valacyclovir daily or 800 mg acyclovir twice daily.8 In a survey from 2012,27 56% of corneal specialists reported that they believed oral antiviral prophylaxis was effective, and that number increased to 71% in a 2019 survey.22 In a retrospective study by Miserocchi and colleagues, 35% of eyes affected by HZO and 39% of eyes affected by HSV had fewer episodes of inflammation with use of low-dose antiviral prophylaxis.35 As this decrease in HSV recurrence was similar to that found in the Herpetic Eye Disease Study,36 it is possible that low-dose, suppressive antiviral therapy could also work for HZ. However, there are no further data to support whether oral antiviral prophylaxis is effective in reducing the anterior segment complications associated with HZO.
The ongoing Zoster Eye Disease Study (ZEDS) (Clinicaltrials.gov: NCT03134196)37 is a prospective, multicentre, randomized controlled trial that seeks to determine if prolonged suppressive antiviral therapy (1 g valacyclovir daily) reduces anterior segment complications of HZO. Specifically, the primary endpoint is delay in time to first occurrence by 12 months of new or worsening dendriform epithelial keratitis, stromal keratitis (with and without ulceration), endotheliitis and iritis. Secondary endpoints include evaluating the treatment effect on primary endpoints 6 months after discontinuation of therapy to determine if there is a long-term effect. The impact of this therapy on PHN will also be assessed. The results of ZEDS will help establish standard of care for the role of prophylactic therapy to prevent HZO complications of the anterior segment. Participation in ZEDS is encouraged.38
Conclusion
HZ is a complex disease that can have multiple ocular manifestations that vary in severity. The treatment of HZK centres on oral and/or topical antivirals for active epithelial disease and topical steroids for immune-mediated stromal and endothelial disease.22–24,27 HZK is often recurrent or chronic, sometimes complicated by NK, and can ultimately lead to stromal scarring or endothelial dysfunction, possibly requiring surgery.8 Vaccination is an effective method of decreasing the incidence of HZO.21 Other ophthalmic manifestations, such as glaucoma, and systemic issues, such as PHN, should be co-managed with appropriate specialists.8 Management of chronic and recurrent HZK disease is challenging, and currently there is little evidence to guide treatment with antiviral medications. The results of the ongoing ZEDS trial will help establish the standard of care.3