Advanced age-related macular degeneration (AMD) is often bilateral and most prevalent in patients over age 60.1,2 AMD is considered end-stage when visual impairment resulting from bilateral central scotomas and/or disciform scars is moderate to profound.
Treatment options for patients with end-stage AMD are extremely limited. As a result, patients with end-stage AMD have a severely reduced quality of life (QoL), become heavily dependent on caregivers,3 and often suffer from stress and depression.4–6 The implantable miniature telescope (IMT; VisionCare Ophthalmic Technologies, Saratoga, CA, US), a prosthetic specifically engineered for patients with end-stage AMD, is designed to address these issues. This device is indicated for monocular implantation to improve vision in patients at least 65 years old who have stable severe to profound vision impairment (best-corrected visual acuity of 20/160 to 20/800) caused by bilateral central scotomas associated with end-stage AMD.7
Our experience with the IMT in the real-world setting is consistent with the clinical utility the prosthesis demonstrated in clinical trials8–11 and has reinforced the need for precise surgical technique, careful patient selection, and multidisciplinary postoperative rehabilitation in order to achieve successful outcomes and high patient satisfaction. The role and contribution of the anterior segment surgeon cannot be overlooked and, therefore, will be the primary focus of this review.
The first implantable prosthetic for late-stage age-related macular degeneration
The IMT is a fixed-focus, monocular prosthetic telescope incorporating ultraprecise, quartz glass, wide-angle Galilean micro-optics. The device is placed in the capsular bag concurrent with cataract surgery in the most qualified eye.8,9 The IMT can provide 2.2x or 2.7x magnification of the central visual field. This telephoto effect enhances visual detail in the central field by reducing the size of the scotoma relative to the objects being viewed. One distinguishing feature is that the implant follows natural eye movement and requires no external devices or head motion to function.
The IMT does require at least 3–4 months of postoperative rehabilitation and a strong commitment from the patient and their support network in order to be successful. Outcomes vary, but the device may enhance near and distance vision with standard eyeglasses and handheld magnifiers as needed, thus improving the patient’s ability to read, prepare food, recognize faces, watch television, and overcome the isolation associated with low vision.8 Although the IMT restricts peripheral vision in the implanted eye, the effect may be ameliorated by eye movement and with compensation by peripheral vision in the non-implanted eye, supporting orientation and safe mobility.
Surgical implantation
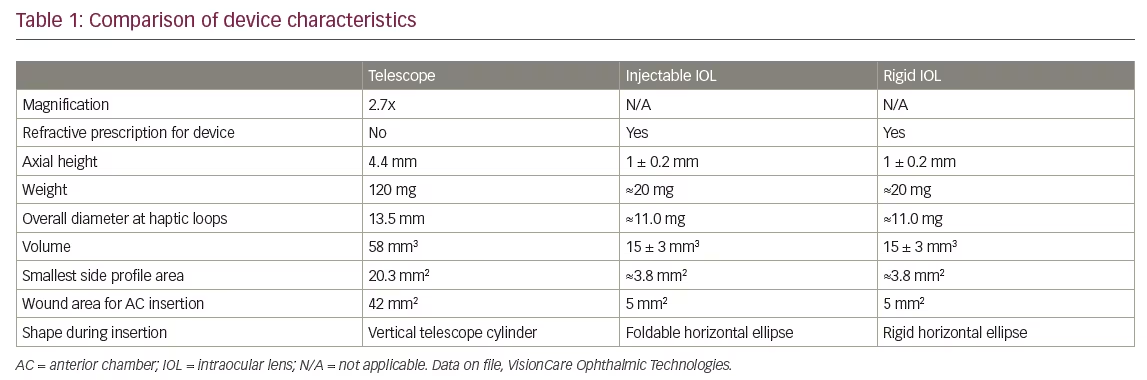
Implantable miniature telescope surgery is similar to extracapsular cataract extraction, with some key differences. Generally, the cataract can be removed by the surgeon’s most comfortable small incision technique, but because the IMT is bigger and heavier than conventional intraocular lenses (IOLs), a larger incision is needed to implant the IMT. A summary of device characteristics (Table 1) highlights the differences in dimensions between the IMT and commercially available IOLs.
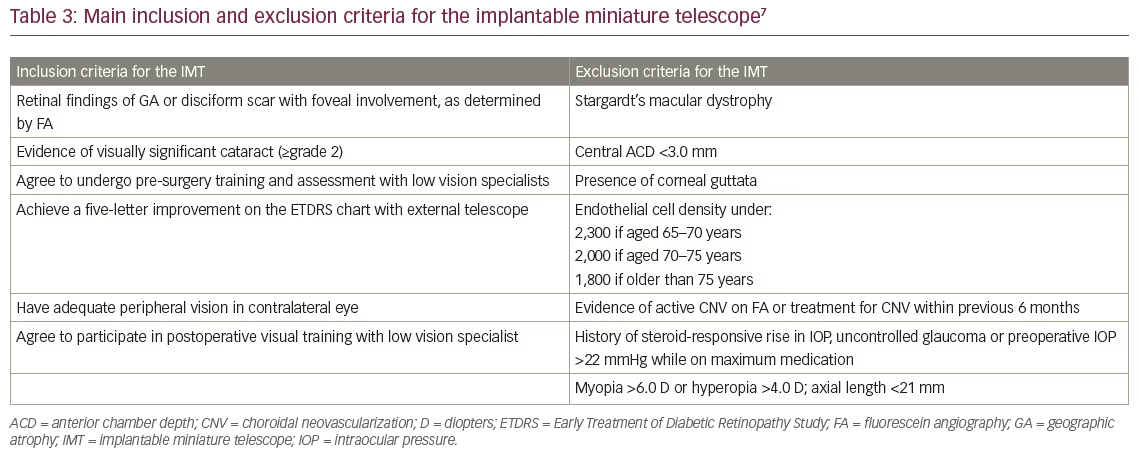
In addition, although the surgical technique does not differ substantially from traditional IOL implantation, there are additional exclusion criteria for the IMT. Patients are generally excluded from IMT implantation consideration if they have already undergone surgery in the proposed eye, if there is poor peripheral vision in the contralateral eye, if there is uncontrolled glaucoma, or if the patient is prone to rubbing their eyes.
Evidence from clinical trial data
The IMT was approved by the US Food and Drug Administration (FDA) in 2010 based on data from the IMT-002 study. In 2015, IMT approval was expanded to include patients 65 years or older.8,10 We will only briefly review the data, as these results have been previously published.
At 1-year follow-up, 67% of IMT-implanted eyes (206/217 implanted/enrolled; 11 aborted cases) had 3 lines or better improvement in best-corrected distance visual acuity (BCDVA), compared to 13% of control eyes (p<0.0001).10 More than half of patients (53%) had 3 lines or better improvement in both BCDVA and best-corrected near visual acuity (BCNVA). The mean improvement in BCDVA and BCNVA was 3.5 lines and 3.2 lines, respectively, compared to 0.8 lines and 1.8 lines in fellow eyes (p<0.0001). At 2 years, 59.5% of implanted eyes had an improvement of 3 lines or better compared to 10.3% of fellow eye controls (p<0.0001).11
Endothelial cell density (ECD) changes following IMT implantation were comparable to the remodeling of the endothelium seen with conventional IOL implantation.5 Changes in ECD stabilized over time, with a chronic annual loss of about 3%, and there was no indication of continued endothelial trauma.
Beyond QoL measures, data shows patients with the IMT experienced a 12.5% QoL gain from a procedure that was determined to be cost-effective by conventional standards.12
Continual disease progression may lead some patients to receive anti-vascular endothelial growth factor (anti-VEGF) intravitreal injections post-IMT implantation. There is at least one published case report of a patient who developed recurrent choroidal neovascularization (CNV) after implantation and received an intravitreal injection; these can be completed successfully and safely by accommodating the dimensions of the implant and monitoring with ocular coherence tomography imaging.13
The rehabilitation process
Patients with the IMT must commit to and undergo postoperative occupational therapy in order to recognize and maintain the magnified image and develop competencies in viewing skills (fixation, steady gaze, tracking, and telescope implant eye image persistence for activities of daily living), visual motor integration and writing skills, reading with the implant eye, and mobility skills with the fellow eye.
One such rehabilitation program is CentraSight. The CentraSight postoperative treatment program includes low-vision optometrists and occupational therapists who prescribe spectacles and help patients learn to use the IMT to the best of their ability. Low-vision optometrists and occupational therapists assist patients with the IMT in locating objects of interest in their field of view with the implant eye, identifying objects, following moving objects, following paths between two objects, as well as general navigation (e.g. walking around).
Our clinical experience with the implantable miniature telescope
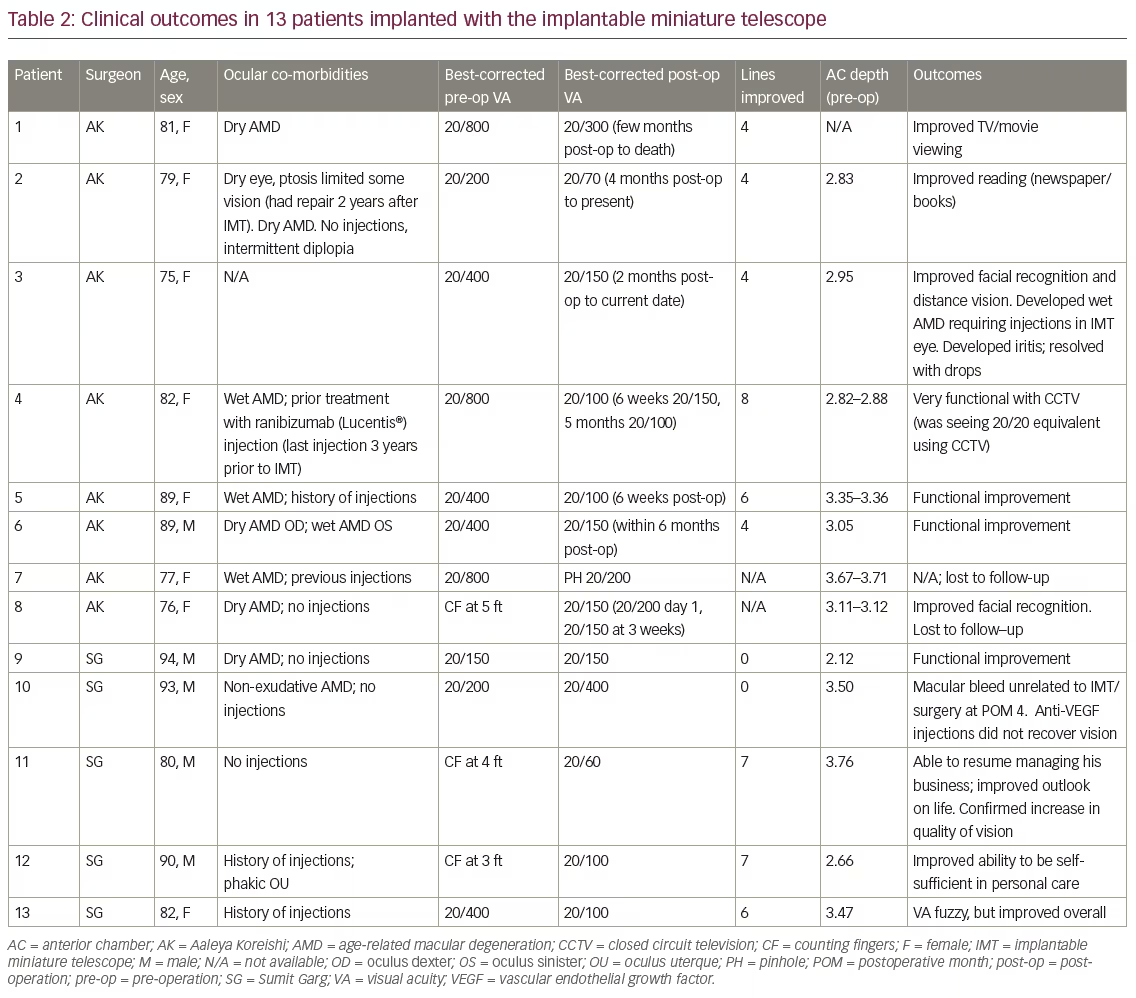
The remainder of this paper will discuss the visual acuity and anecdotal QoL outcomes we achieved in our first 13 patients implanted with the IMT where some postoperative follow-up is available. A summary of this experience is provided in Table 2. In short, we lost two eyes to follow-up; of the remaining 11 eyes, nine (82%) gained 4 lines of vision and two (18%) did not gain any lines of vision.
Implanted-eye selection and patient eligibility was determined by phakic status, ocular comorbidities, patient preference, and low-vision specialist recommendation based on best-seeing eye evaluation with the External Telescope Simulator (ETS). Table 3 lists the main inclusion/exclusion criteria. The IMT is implanted in the better-seeing eye as it aids central vision.8
There was a wide variety of visual acuity line gains that ranged from 0 to 8 in our real-world cohort. The majority of patients achieved 4 or more lines of BCDVA; anecdotal stories indicated a substantial improvement in QoL even for those who achieved no vision gains. This was also true in our oldest, most vulnerable patients. Perhaps most dramatically, one female patient went from counting fingers at 5 feet preoperatively to 20/150 1-month postoperatively. The patient’s husband wept as he described how she could see his face for the first time in years. Although some patients did not demonstrate a measurable improvement in vision, all patients had a subjective improvement in vision that led to perceived improved visual quality and initial satisfaction with the surgery.
Adverse events associated with IMT implantation were consistent with those reported in the literature and included intermittent diplopia, ptosis, and iritis. At least two patients have received anti-VEGF injections with the IMT in place, including one patient who developed macular bleed deemed to be unrelated to the IMT implantation. That patient’s vision declined from 20/200 preoperatively to 20/400 postoperatively. Anti-VEGF injections did not recover vision. However, in patient three, despite progression to wet AMD requiring injections, improvement was maintained. This adds to our general knowledge of AMD in that progression can still occur even after surgical intervention and continued monitoring and treatment are warranted.
Continued follow-up will reveal whether these results are stable over time, particularly in patients whose AMD progresses or who experience other ocular pathologies.
Key insights for the implantable miniature telescope surgeon
Our experience with the IMT suggests the optimal outcomes with the prosthesis are dependent on successful management of pre-, intraoperative, and postoperative phases. Although the surgeon is chiefly responsible for the implantation procedure itself, he or she must support the other team members in patient selection, assessment, and rehabilitation, and help guide the patient through the treatment process.
Managing patient expectations
Managing patient expectations from the outset is essential to optimizing postoperative rehabilitation and patient satisfaction. Patients should understand that the IMT is not a “cure” for their end-stage disease, and that, in fact, their AMD could progress. It is important to stress the differences in recovery between cataract surgery and IMT implantation; patients will likely experience stepwise improvements over 3 to 4 months, not drastic improvement over days or weeks. They need to understand that the IMT is not a “plug and play” device. It takes time for them to learn how to use their new vision. Emphasizing QoL improvements rather than actual Snellen letter gain may be more relevant for the patient and provide encouragement to diligently pursue and continue postoperative rehabilitation. We noted an increased ability to read and write, watch TV, and recognize faces in the majority of our patients, but we did need to caution patients that they would be very unlikely to regain their ability to drive.
Patient selection and surgical criteria
Evaluating the patient with an ETS, used in both preoperative training and visual acuity testing, enhances the ability of the low-vision specialist to determine if a patient is a suitable candidate. We have found patients should be able to achieve improvements of at least five letters on the ETDRS chart with the ETS in the eye to be implanted in order to proceed with surgery.
We believe the cataract surgeon should assess a prospective patient once the retina specialist has determined there is no active wet AMD and the patient would not benefit from anti-VEGF treatment; ideally this should be before referral to the low-vision optometrist and occupational therapist. Determining that a patient is a good surgical candidate at this stage (by confirming appropriate age, corneal health, sufficient anterior chamber depth, adequate pupillary dilation, absence of contraindications such as Fuch’s endothelial dystrophy and pseudoexfoliation syndrome, and reasonable expectations, for example) can expedite the process and reduce the number of premature assessments (including use of the ETS) and preparations. The surgeon should perform a standard cataract evaluation with the addition of specular microscopy; increased chair time should be expected with the patient, family members, or other caregivers than would ordinarily be necessary with conventional lens replacement surgery.
Postoperative anterior chamber depth (ACD) should be greater than 3 mm per the manufacturer’s directions for use;7 however, surgeons can exercise their best clinical judgment as we have successfully performed many of these cases on patients with anterior chambers less than 3 mm, particularly if the ACD is impacted by a fairly thick cataract. Surgeons can expect some deepening of the ACD by removal of the cataract alone, and in normal/longer eyes, there is usually sufficient space for safe implantation of the IMT. Additionally, many of these patients present having already undergone cataract surgery in their fellow eye. In these cases, surgeons can gauge what the pseudophakic ACD will be and make an appropriate judgment.
Patients must understand their role in postoperative rehabilitation. Successful outcomes depend on the patient’s ability and commitment to regularly attend occupational therapy appointments. Patients who did best had a strong support network and consistent follow up and practice with occupational therapy.
Patients who achieve the best long-term results are those who understand the risk/benefit ratio, are willing to work hard at rehabilitation, and have a strong network of practical and emotional support. Patients who will have trouble keeping occupational therapy appointments may not be good candidates, nor is a patient who has unreasonable expectations or who is looking for a “cure” for AMD.
Surgical technique
The literature is clear that safety outcomes are directly related to surgical technique and experience.8,10 The surgical technique has been previously described, and our experiences add to the literature.9 In our hands, we found an increased surgical time of about 30 minutes. We found a 7 mm capsulorhexis, coupled with an 11 to 13 mm scleral incision (or a 10 to 12 mm limbal incision) and placing those incisions as close to the limbus (short scleral tunnel) as possible will make the IMT insertion a bit easier.
We prefer to use specially-designed forceps (Duckworth & Kent) to grasp the IMT. We believe the angulation in the forceps permits easier implantation of the IMT into the capsular bag. Handle the device by the carrier plate, avoiding any contact with the haptics or glass cylinder. At insertion, the cornea must be lifted 5 to 6 mm maximally to allow the leading haptic of the IMT to enter the bag at a 45-degree angle to the horizontal plane. Avoid bending the cornea, and take measures to protect the endothelium, including use of ophthalmic viscosurgical devices (OVD). In our hands, we found ample use of the OVD (cohesive in the bag, dispersive in the anterior chamber and on the IMT), coupled with a modified soft-shell approach (dispersive OVD against the endothelium and a cohesive behind), can effectively manage the anterior chamber.
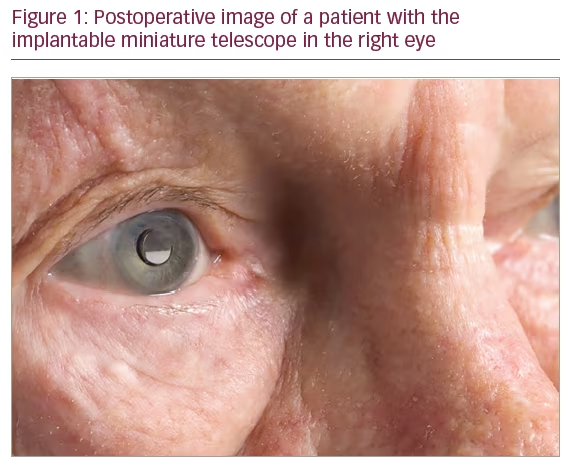
Figure 1 shows a patient with the IMT implanted.
Postoperative management
Postoperatively, the anterior surgeon typically needs to implement a follow-up visit regimen on day 1, at weeks 1 to 2, and at month 1. At that last visit, selective suture removal can begin, based on corneal astigmatism. Engagement with the low-vision team as soon as possible after the initial surgery recovery allows the patient to learn how best to use their IMT optimally. Generally, active rehabilitation with the low-vision team is between 6–10 sessions over a 3–4-month period, beginning after the dilation drop regimen has been completed during the initial recovery period (7–10 days postoperatively). Glasses prescription may be given but may change depending on corneal astigmatism and suture removal. Patients may begin vision rehab even without a final spectacle prescription.
Incorporating the implantable miniature telescope into a comprehensive treatment algorithm for age-related macular degeneration
The opportunity to improve functional vision in patients with end-stage AMD for the first time necessitates a re-examination of the treatment paradigm for the disease. Retina specialists, anterior segment specialists, and low-vision rehabilitation specialists will comanage these patients, with low-vision specialists undertaking the bulk of the postoperative patient management once the optometrist has fitted the patient for new glasses.
Conclusion
The IMT offers a unique opportunity to restore some central vision to patients whose sight has been impaired by end-stage AMD. Consistent with published literature, our experience with the prosthesis suggests that real-world patients can achieve significant improvement in best-corrected visual acuity, functional vision, and QoL. Careful surgical technique and experience with implantation, as well as engagement in patient selection, counseling, and rehabilitation, support postoperative outcomes that meet or exceed the best results already found in previous studies.







