Macular hole (MH) and vitreomacular traction (VMT) can result in disruption of the vital area of contact between the photoreceptors and the nourishing retinal pigment epithelium (RPE).1–3 Optical coherence tomography (OCT) has become the gold standard tool for diagnosing and managing these conditions.3–5 OCT is often used for monitoring surgical outcomes in patients with MH and VMT, to ensure that the hole has closed or VMT has released. However, in some patients, despite achieving the desired anatomical outcome, disruptions are seen at the level of the photoreceptor bands on OCT (EZ and IZ). In other patients, despite less-than-expected visual improvement, no abnormality is seen on OCT. In fact, there are inconsistencies across studies comparing OCT findings to visual acuity.5–13 While the integrity of the photoreceptors is of greatest interest in both, the limited lateral resolution of OCT precludes quantitative assessment of photoreceptors by enumeration and cellular integrity. Given that photoreceptor structure may hold prognostic value for patients with MH and VMT after surgical intervention, there is a requirement to develop advanced imaging approaches in these patients. Adaptive optics scanning light ophthalmoscopy (AOSLO) is capable of correcting the eye’s monochromatic aberrations, enabling in vivo imaging of the photoreceptor mosaic with cellular resolution.14,15 AOSLO has been used to image patients with EZ/IZ abnormalities due to ocular trauma,16 following surgical closure of idiopathic MH17–19 and eyes with epiretinal membrane (ERM).20 A general finding in previous patients was the consistent presence of ‘dark areas’ within the central macula; previously, it had been suggested that these areas were due to cone cell loss.17,19 However, conventional confocal AOSLO suffers from a significant limitation – detection of photoreceptor cells relies on proper waveguiding of light by structurally intact cells.21 Thus, loss of the reflective signal on confocal AOSLO in these patients may be due to damaged as opposed to absent cone cells. Resolving this matter is critical to realising the full potential of advanced imaging for evaluating surgical outcomes and establishing realistic expectations
for patients. Recently, a non-confocal technique called ‘split-detector’ AOSLO was developed, which detects multiply scattered light from the photoreceptor inner segments.21 This permits visualisation of cones in a manner that appears to be independent of their ability to waveguide light. It has been shown, in various patient populations, that areas appearing dark on confocal AOSLO can still retain an intact inner segment structure.18,21 Here we used split-detector AOSLO to examine dark areas observed on confocal AOSLO in patients following surgical intervention for MH or VMT, with the goal to more accurately characterise the status of residual cone structure in these patients. We found that dark areas within confocal AOSLO imagery often contain remnant cone inner segment structure. The degree to which these cones are functional remains unclear, but the imaging approach used here provides an important tool for examining the structure– function relationship in MH, VMT and related conditions.
Methods
Human Subjects
This research followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board at the Medical College of Wisconsin. Informed consent was obtained and documented for all subjects prior to imaging. Six subjects (two male; four female) with MH and VMT lesions participated in spectral domain optical coherence tomography (SD-OCT) and AOSLO imaging. Seven eyes, five with MH and two with VMT, were included in this study. The age (average = 66 years), time post-intervention, pre-intervention best-corrected visual acuity (BCVA), pre-imaging BCVA and lens status varied between subjects (see Table 1). Axial length was measured in all eyes using an intraocular lens (IOL) Master (Carl Zeiss Meditec, Dublin, CA, US) to facilitate accurate lateral scaling of retinal images.
Spectral Domain Optical Coherence Tomography
Images were obtained using SD-OCT (Bioptigen, Inc., Durham, NC, US) just prior to AOSLO imaging. Linear B-scans (1,000 A-scans/B-scan; 100 repeated B-scans) were averaged as previously described.22 In addition 3 x 3 mm (1,000 A-scans/B-scan; 400 B-scans/volume) and/or 7 x 7 mm (1,000 A-scans/B-scan; 250 B-scans/volume) volumetric scans were acquired from each eye.
Adaptive Optics Scanning Light Ophthalmoscopy
AOSLO imaging included simultaneous collection of images using both confocal and split-detection channels. Full system details have been previously described.14,21 A 790 nm superluminescent diode was used for imaging, with the field of view of each image subtending either 1 x 1° or 1.5 x 1.5°. An internal fixation target was used to guide fixation and allow acquisition of images from different retinal locations. Individual image sequences at each fixation location contained 150 frames. Each image sequence was ‘desinusoided’ to correct distortions introduced by the sinusoidal movement of the resonant scanner. Next, a reference frame within each image sequence having minimal distortion and maximal sharpness was identified, and registration of frames within a given image sequence to their respective reference frame was performed using a previously described strip-registration algorithm (Python; Python Software Foundation).23 A final image for each image sequence was created by averaging together the frames with the highest normalised cross-correlation to the reference frame. The split-detector images were processed in parallel, using the same registration parameters applied to the confocal images. The final split-detector and confocal images were both montaged using commercial software (Adobe Photoshop; Adobe Systems Inc., Mountain View, CA, US).
Results
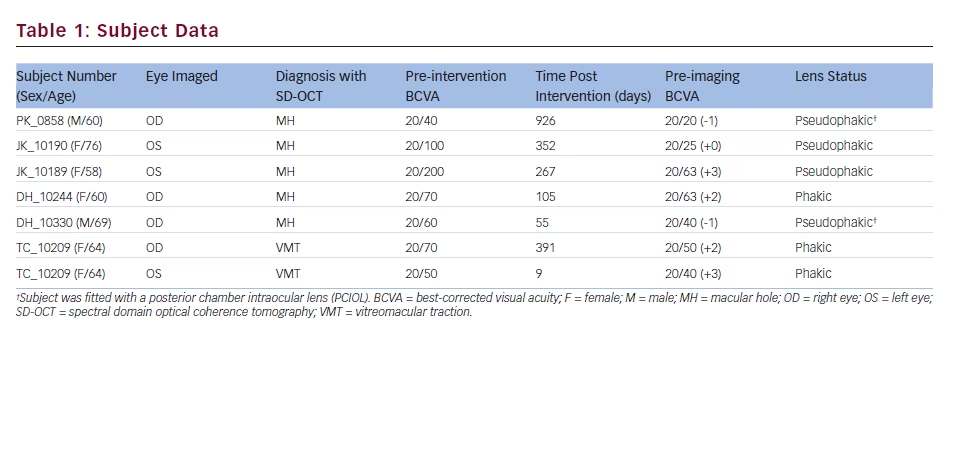
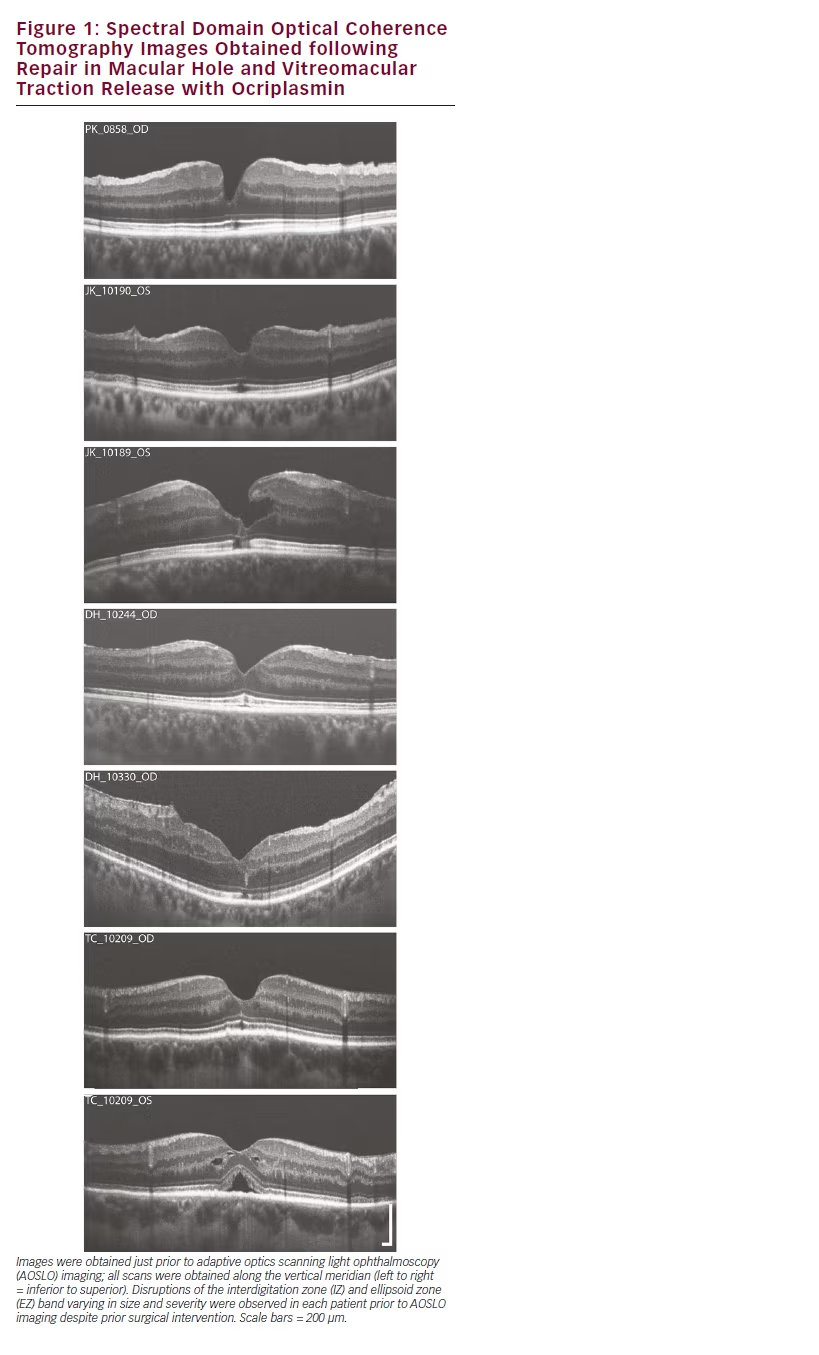
Variable disruption of the photoreceptor bands (EZ and IZ) on OCT was observed in all seven eyes (see Figure 1). In all but one subject, confocal AOSLO montages showed large areas of reduced reflectivity, consistent with previously described dark areas in similar patients.18–20 The size and location of these dark areas more closely matched the IZ disruption on OCT as opposed to the EZ disruption, consistent with previous findings examining confocal AOSLO lesions in other conditions.18 The confocal AOSLO montage in the left panel of Figure 2 illustrates a typical lesion observed in our subjects. The remaining MH subject (DH_10330) showed multiple focal regions of reduced reflectivity in his confocal AOSLO montage along with areas of ambiguous hyperreflectivity.
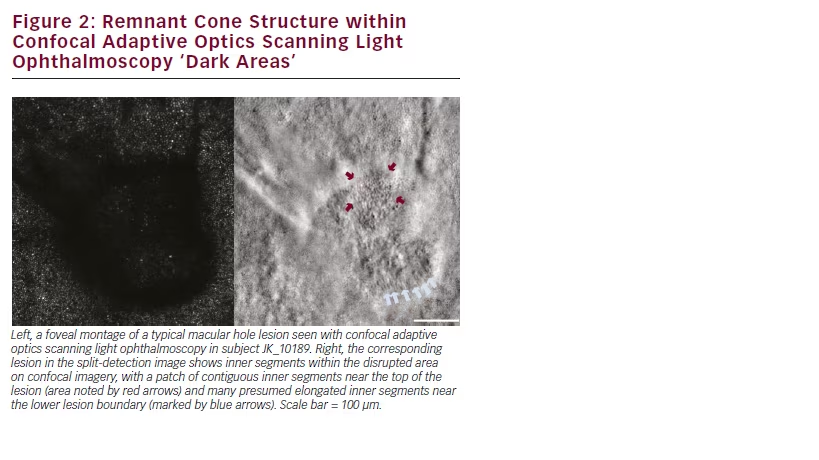
When imaged using split-detector AOSLO, numerous cone inner segments are observed within the lesion boundaries, as seen in the right panel of Figure 2. Using split-detector AOSLO, remnant inner segment structure was observed within the dark areas on the confocal AOSLO montages across all subjects with repaired MH or released VMT that we imaged (see Figure 3). Often there were patches of contiguous cone inner segments (TC_10209 (OS), PK_0858, JK_10189, DH_10244, DH_10330), while in some subjects the remnant cone population was sparsely arranged (TC_10209 (OD) and JK_10190). As cone visibility on confocal AOSLO is believed to require intact inner and outer segment structure,21 the discrepancy between the confocal and split detection images shown here is best explained by disrupted outer segment structure in these subjects.
While cones normally appear as bright circular structures on confocal AOSLO, not all such structures are indeed derived from cones. For example, when viewed on confocal AOSLO, the RPE can produce similar-appearing structures.24 In addition to the numerous dark areas seen in the confocal AOSLO imagery from DH_10330, we observed regions of ambiguous hyperreflectivity (see Figure 4) on confocal AOSLO. While some of these appeared to correspond to remnant cone inner segment structure on split-detector AOSLO (consistent with intact cones), others did not (Figure 4). Inspection of the OCT reveals hyperreflective material within the ONL in this subject (see Figure 1), which could be contributing to the granular reflective structure seen on

the confocal AOSLO. This subject illustrates the complementary nature of OCT, confocal AOSLO and split-detector AOSLO.
Discussion
This case series demonstrates that split-detector AOSLO can provide unique structural information in patients with history of VMT and MH. In each case, remnant inner segment cone structure was present on split-detection imagery where corresponding confocal AOSLO showed a lack of waveguiding photoreceptors. This finding directly contradicts the previous assertion that dark areas in MH lacked any photoreceptor structure.17,19 In addition, some areas within confocal lesions containing bright reflective structures (apparent cones) did not show inner segment structure in the corresponding split detector image (DH_10330). While the split-detection channel has slightly lower resolution that could interfere with the ability to resolve the small inner segments in the central fovea, the visibility of nearby inner segments suggest these areas are truly devoid of cone structure. Together, these observations highlight the fact that not all bright circular structures within confocal AOSLO images originate from cones, and that split-detector AOSLO imaging can serve a valuable role in interpreting the degree of residual cone structure in these cases.
We previously showed that the appearance of the cone mosaic using confocal imaging can change over time in patients following MH closure, namely that cones were seen to re-emerge within the dark lesions seen initially.18 In that study, we analysed the surrounding cone mosaic and found no significant differences across the imaging sessions, arguing against active ‘migration’ of nearby cones into the previously observed dark lesion.18 Specifically, we hypothesised that the visualisation of cones in long-term follow-up imaging was due to recovery of cone outer segments within the dark lesion. A similar finding was observed in patients with macular telangiectasia, where cones become visible using confocal AOSLO in areas that appeared dark in a previous imaging session.25 The data obtained here using splitdetection AOSLO provide anatomical support for these observations, showing that inner segment structures can persist within dark lesions on confocal AOSLO. This same finding was recently reported in patients with other retinal conditions,18,26,27 and argues for continued longitudinal examination of patients with various retinal conditions using both confocal and split-detector AOSLO.
Limitations in this study included a small sample size as well as the lens status of our subjects. Four of the eyes imaged were pseudophakic, which is a common finding due to the increased incidence in cataract development following surgical repair of MH and VMT. Pseudophakic eyes typically have poorer resolution in AOSLO imaging due to the smaller optical aperture provided by intraocular lenses. In addition, the imaging process can be difficult in patients who have lesions at their fovea; we found that most of our subjects were unable to focus reliably on the internal fixation target within the AOSLO system. To overcome this, we obtained multiple images at each intended fixation locus and returned frequently to image the fovea throughout the session, thus increasing the probability that we would have adequate retinal coverage to be able to construct a montage following image processing. However, these images are generally of lower quality than that typically achievable in phakic patients. Another limitation is the qualitative nature of the analysis. As split-detector AOSLO is quite new, there are little to no normative data with this technique and significant work remains to develop reliable analytical approaches for these new images. Nevertheless, the results shown here provide motivation for continuing to explore the complementary nature of split-detector AOSLO and conventional confocal AOSLO across other retinal conditions.
Conclusion
Images from confocal and split-detector AOSLO are useful in identifying photoreceptor structure within lesions that are commonly observed using OCT in patients with MH and VMT. While confocal AOSLO imaging relies on intact cone waveguiding, split-detector AOSLO enables visualising cones in a manner that appears independent of their waveguide reflectivity. The results of this study imply that analyses based solely on assessment of cone structure using confocal AOSLO imagery are likely to be incorrect and should be re-evaluated. While this new imaging approach provides valuable structural insight into the repair process of MH and VMT pathology, we cannot say anything about the functional status of these cones. Moving forward, it will be important to look at results like these with tools such as AO-guided microperimetry (AOMP),25 which overcome the inherent spatial redundancy in the stimuli employed in conventional functional assays available in the clinic.







