Stevens-Johnson Syndrome
Stevens-Johnson syndrome (SJS), an acute inflammatory vesiculobullous reaction of the skin and mucous membranes, was first described in 1922.1 Stevens and Johnson, both paediatricians, encountered two boys seven and eight years of age who manifested an extraordinary, generalised skin eruption, persistent fever, inflamed buccal mucosa and severe purulent conjunctivitis resulting in severe visual disturbance. They carefully ruled out drug ingestion as a causative factor of the skin eruption. Subsequently, paediatricians reported that SJS was associated with infectious agents such as Mycoplasma pneumoniae2 and had a viral aetiology involving herpes simplex virus, Epstein-Barr virus, cytomegalovirus and varicella zoster virus.3
In contrast, dermatologists claimed that more than 100 drugs were involved in eliciting SJS and its severe variant, toxic epidermal necrolysis (TEN).4 They cited life-threatening severe adverse drug reactions characterised by high fever, rapidly developing blistering exanthema of macules and target-like lesions accompanied by mucosal involvement and skin detachment.4,5
Formerly, erythema multiforme (EM), SJS and TEN were accepted as being part of a single EM spectrum; however, a retrospective analysisof the type and distribution of the skin lesions and the extent of epidermal detachment identified EM major and SJS/TEN as two distinct clinical entities that differed with respect to their histopathological changes and aetiology.6 The annual incidence of SJS and TEN has been estimated as 0.4 to one and one to six cases per million persons, respectively;6,7 the reported mortality rate is 3 and 27%, respectively.8 Although rare, these reactions carry high morbidity and mortality rates and often result in severe and definitive sequelae such as vision loss. The pathobiological mechanisms underlying the onset of SJS/TEN have not been fully established. The extreme rarity of cutaneous and ocular surface reactions to drug therapies led us to suspect individual susceptibility.
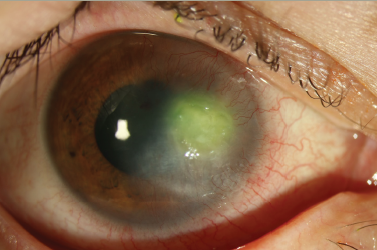
In the acute stage, SJS/TEN patients manifest severe conjunctivitisand corneal/conjunctival epithelial defects with vesiculobullous skin lesions. In the chronic stage, ocular surface complications such as conjunctival invasion into the cornea due to corneal epithelial stem cell deficiency, symblepharon, ankyloblepharon and, in some instances, keratinisation of the ocular surface persist despite healing of the skin lesions (see Figure 1).9 We documented elsewhere that more than 95% of patients with SJS/TEN with ocular surface complications had lost their fingernails in the acute or subacute stage, and that some continued to have transformed nails even after healing of their skin lesions (see Figure 2).10,11 SJS/TEN is one of the most devastating ocular surface diseases; it leads to corneal damage and vision loss. The reported incidence of ocular complications in SJS/TEN is 50–68%.7,8
According to a consensus classification proposed in Europe and North America, in bullous EM less than 10% of the body surface area (BSA) is detached, and localised typical or raised atypical targets are present. In SJS, less than 10% of the BSA is detached and there are widespread erythematous or purpuric macules of flat atypical targets. In overlapping SJS/TEN, BSA detachment ranges from 10 to 30% and there are widespread purpuric macules or flat atypical targets. Lastly, TEN with spots involves more than 30% of the BSA and there are widespread purpuric macules or flat atypical targets, while in TEN without spots BSA detachment is greater than 10% and large epidermal sheets but no purpuric macules are present.4,6 In Japan, a diagnosis of SJS is made in cases presenting with less than 10% BSA detachment, widespread blistering exanthema of macules and atypical target-like lesions accompanied by mucosal involvement;12 TEN is diagnosed when BSA detachment exceeds 10%.12
Dermatologists tend to see patients with SJS/TEN in the acute stage; in contrast, most patients encountered by ophthalmologists present in the chronic stage of SJS/TEN. In fact, of our 71 SJS/TEN patients, 55 (77%) were in the chronic stage, seven (10%) were in the sub-acute stage and the remaining nine (13%) presented with acute-stage SJS/TEN when they first reported to our hospital. In patients in the chronic stage of SJS/TEN, the differential diagnosis of SJS or TEN may be difficult because the vesiculobullous skin lesions present in the acute stage will have healed. Thus, ophthalmologists usually diagnose both SJS and TEN as SJS in a broad sense.
Our diagnosis of SJS/TEN (SJS in a broad sense) was based on a confirmed history of acute-onset high fever, serious mucocutaneous illness with skin eruptions and involvement of at least two mucosal sites, including the ocular surface.9–11,13–16
Stevens-Johnson Syndrome and Human Leukocyte Antigen
In 1982, the ophthalmologist Mondino and colleagues first erformed human leukocyte antigen (HLA) analysis in patients with SJS.17 Their examination of 15 Caucasian patients with SJS with ocular involvement found that the HLA-Bw44 antigen, a subgroup of HLA-B12, was significantly increased in these patients compared with a control Caucasian population of 411 individuals 66.7 versus 20.4%; p<0.001, corrected p [pc] <0.034).17 In this study, the onset of SJS with ocular involvement was associated with putative viral syndromes or the administration of drugs.17
The second report involving HLA analysis in SJS was published by the dermatologist Roujeau and colleagues in 1986.18 Their study of 45 French SJS/TEN patients whose disorder was clearly druginduced showed that the frequency of the HLA-B12 antigen was significantly increased compared with a French control population comprising 66 individuals (57.7 versus 25.7%; p<0.001, pc<0.04).18 The causative agents were non-steroidal anti-inflammatory drugs NSAIDs) in 25 patients (56%), including 18 who had taken oxicam derivatives, and sulphamides in 14 (31%).18sup> The association with HLA-B12 was most pronounced in patients treated with sulphonamide and oxicam NSAIDs. In another study, the same group found that the frequency of the HLA-B12 antigen was significantly increased in 44 surviving French TEN patients compared with a French control population.19 In 1996, Power et al. reported that the frequency of the HLA-DQB1*0601 antigen was significantly increased in 23 Caucasian SJS patients with ocular complications compared with 175 Caucasian controls (17 versus 3%; p=0.0017, pc<0.05; odds ratio [OR] 7.2).20
We subsequently examined HLA-class I (HLA-A, -B, -C) antigens in 40 Japanese SJS/TEN patients with ocular complications.13 We found that the carrier frequency of the HLA-A*0206 antigen was significantly higher in these patients compared with 113 Japanese controls (47.5 versus 15.0%; p=0.00003, pc<0.0005; OR 5.1) and that there was a negative association with HLA-A*1101.13 Furthermore, we studied HLA class I and II (DRB1 and DQB1) gene polymorphisms in 71 Japanese patients with SJS/TEN with ocular complications.16 Again, we found that HLA-A*0206 was strongly associated with SJS/TEN with ocular complications; there was no association with HLA-DQB1*0601.16 The onset of SJS with ocular complications was associated with putative viral syndromes or the administration of drugs,13,16 a finding that coincided with that of Mondino.17 Although the HLA-B12 antigen was significantly increased in Caucasian SJS patients,17–19 we found no association with HLA-B12 in Japanese SJS patients,13,16 probably because in Caucasians the HLA-B12 antigen is primarily coded by HLA-B*4402 whereas in the Japanese population it is almost exclusively coded by HLA-B*4403.21 In contrast, HLA-A*0206, strongly associated with SJS/TEN with ocular complications in Japanese individuals, is absent in Caucasians. We detected no significant association between SJS/TEN and HLADQB1* 0601,16 although HLA-DQB1*0601 was associated with ocular complications in Caucasian SJS patients.20
Thus, our findings suggest strong ethnic differences in the association of SJS/TEN with HLA (see Table 1). Because SJS/TEN is rare and probably has a complex genetic inheritance background, specific combinations of genes and certain environmental factors may be required for the manifestation of this rare phenotype.
Drugs and Human Leukocyte Antigen
The association between HLA and drug-induced severe cutaneous adverse reactions including SJS and TEN has been reported. The HLAB* 1502 allele showed a very strong association with carbamazepineinduced SJS/TEN in the Han Chinese of Taiwan.22 In that study, dermatologists examined 44 patients with carbamazepine-induced SJS. They found that the frequency of HLA-B*1502 was significantlyincreased compared with a carbamazepine-tolerant control group of 101 individuals (100 versus 3%; pc=3.13×10-27; OR 2504) and a control population consisting of 93 normal subjects (8.6%; pc=1.38×10-21; OR 895).22 In supplemental data, they showed that 28 of the 44 patients (63.6%) manifested ocular surface erosion.22 In contrast, no such strong carbamazepine-specific association between HLA-B*1502 and carbamazepine-induced SJS/TEN was found in Caucasian patients.23,24
In our study of 71 Japanese SJS/TEN patients with ocular complications and 113 Japanese controls, we did not detect HLA-B*1502 in either group16 because the allele frequency of HLA-B*1502 is very low in Japanese individuals. We suggest that the carbamazepine-specific association between HLA and carbamazepine-induced SJS may also be specific to certain ethnic groups (see Table 2).
Another Taiwanese study showed that HLA-B*5801 was present in all Han Chinese with allopurinol-induced SJS/TEN and drug-induced hypersensitivity (DIHS).25 All 51 patients with allopurinol-induced severe cutaneous reactions including SJS, TEN and DIHS carried HLA-B*5801 compared with 20 of 135 (15%) allopurinol-tolerant controls (pc=4.7×10-24; OR=580) and 19 of 93 (20%) controls drawn from the general population (pc=8.1×10-18; OR=393.5).25 Of the 51 patients, 21 (41%) manifested ocular surface erosion.25 Lonjou et al.23 also reported an association between HLA-B*5801 and allopurinol-induced SJS/TEN: 15 of 27 European patients (56%) with allopurinol-related SJS/TEN had HLA-B*5801 compared with 28 of 1,822 individuals (1.5%) from a mixed European population (p<10-8, pc<10-6; OR 80).23
Neither our 71 Japanese SJS/TEN patients with ocular complications nor our 113 Japanese controls manifested HLA-B*5801,16 because the allele frequency of HLA-B*5801 is very low in Japanese individuals. None of our 71 patients had allopurinol-related SJS/TEN (Ueta et al., unpublished data). In contrast, the Japanese dermatologist Dainichi and colleagues26 identified three HLA-B*5801 carriers among patients with allopurinol-associated SJS, DIHS and SJS/TEN. These findings suggest that HLA-B*5801 may represent a genetic biomarker for allopurinol-associated SJS/TEN in Japanese patients. Thus, the strong allopurinol-specific association between HLA-B*5801 and allopurinol-induced severe cutaneous adversereactions including SJS, TEN and DIHS may be universal (see Table 2).
Discussion
American ophthalmologists17 and French dermatologists18 have documented that the HLA-B12 (HLA-Bw44) antigen was significantly increased in Caucasian SJS patients. However, in our Japanese study population there was no association with HLA-B12, probably because in Caucasians the HLA-B12 antigen is primarily coded by HLAB* 4402, whereas in the Japanese it is almost exclusively coded by HLA-B*4403.21 In contrast, while HLA-A*0206 was strongly associated with SJS/TEN with ocular complications in Japanese patients,13,16 it is absent in Caucasian populations. Thus, HLA-A*0206 may be related to Japanese ethnicity. These findings suggest strong ethnic differences in the HLA-SJS/TEN association and point to the need for studies in other ethnic populations to obtain a global picture.
With respect to the association between HLA and drug-induced severe cutaneous adverse reactions (SCAR), including SJS and TEN, the association between the HLA-B*1502 allele and carbamazepineinduced SJS/TEN22,24 and between the HLA-B*5801 allele and allopurinol-induced severe cutaneous adverse reactions23,25 has been reported.
In Han Chinese22 but not in Caucasian patients,23,24 there was a strong carbamazepine-specific association between HLA-B*1502 and carbamazepine-induced SJS/TEN. We did not identify HLAB* 1502 in either our Japanese SJS/TEN patients or our controls,13,16 because the allele frequency of HLA-B*1502 is very low in the Japanese. Thus, the carbamazepine-specific association between HLA and carbamazepine-induced SJS may be specific to certain ethnic groups.
An allopurinol-specific association between HLA-B*5801 and allopurinol-induced SCAR was identified in all Han Chinese,25 Caucasian23 and Japanese patients.26 Thus, the strong allopurinolspecific association between HLA-B*5801 and allopurinol-induced SCAR, including SJS, TEN and DIHS, may be a universal phenomenon. Interestingly, none of our 71 Japanese SJS/TEN patients with ocular complications manifested allopurinol-related SJS/TEN (Ueta et al., unpublished data). It is possible that allopurinol-induced SCAR may not elicit serious sequelae on the ocular surface.
Drugs are probably the most widely accepted aetiological factor in SJS/TEN.4,5,27 It is worth noting that SJS/TEN patients often present with prodromata, including non-specific fever, coryza and sore throat, that closely mimic upper respiratory tract infections commonly treated with antibiotics.7,10 The clinical records of our SJS/TEN patients indicated the presence of prodromata.10
Yetiv et al.,7 who published a retrospective analysis of the aetiological factors in 54 SJS patients diagnosed at Johns Hopkins hospital between 1966 and 1976, indicated that drugs and infections were particularly suspect as aetiological agents in SJS. However, they were unable to state unequivocally that drugs were the aetiological factors because the prodromata of SJS include nonspecific fever, coryza, sore throat and malaise – symptoms that closely resemble upper respiratory tract infections commonly treated with antibiotics.7 Consequently, although antibiotics are often suspected to play a role in the manifestation of SJS, they found it difficult to ascertain whether drug treatment induced SJS or whether the prodromata would have developed into full-blown SJS even without the administration of the drugs.7
Of our 71 patients, 55 (77.5%) developed SJS after receiving treatment for the common cold with antibiotics, cold remedies and/or NSAIDs; only four patients (5.6%) progressed to SJS after drug treatment to prevent the occurrence of convulsions. Considering the association between the onset of SJS/TEN and infections, and the opportunistic infection of ocular surfaces by bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and methicillinresistant Staphylococcus epidermis (MRSE),28 we evaluated the possibility of an association between SJS/TEN and a disordered innate immune response.10,11 We postulated that viral infection and/or drugs may trigger a disorder in the host’s innate immune response and that this event is followed by aggravated inflammation of the mucous membranes, ocular surface and skin. We also documented that in Japanese SJS/TEN patients with ocular complications there was an association with TLR3-10 and IL4R polymorphisms.11 Thus, not only genetic factors related to HLA but also innate immunity play important roles in the integrated aetiology of SJS/TEN.
A group of dermatologists reported that allopurinol, uric-acidlowering rugs (17.4%) and anticonvulsant drugs such as carbamazepine (8.2%), nevirapine (5.5%), phenobarbital (5.3%), phenytoin (5.0%) and lamotrigine (3.7%) were commonly associated with SJS or TEN and that cotrimoxazole (6.3%), an antibiotic, was also associated.27 We posit that the SJS/TEN patients seen by dermatologists are not always the same as the SJS/TEN patients consulting opthalmologists.