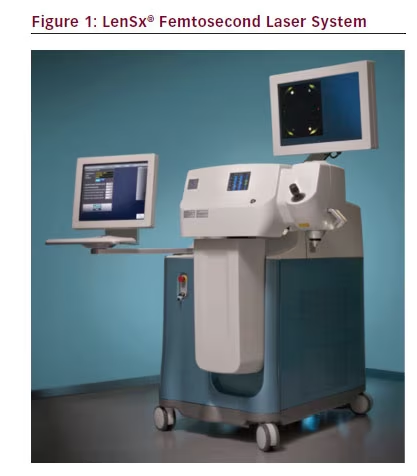
Femtosecond lasers have been successfully used in ophthalmology since 2001,1 most notably in bladeless laser-assisted in situ keratomileusis (LASIK). The high frequency of this laser allows for significantly lower energy to achieve a desired effect; this reduces the potential for collateral tissue damage.2 With modern imaging systems, the laser can be focused to create precise incisions that can be positioned in any desired plane.3 The success of the technology has resulted in the introduction of femtosecond laser systems into cataract surgery, allowing for automation of some of the most critical steps in cataract surgery. The first available laser system approved by the US Food and Drug Administration (FDA) for this application was the LenSx® femtosecond laser system (LenSx Lasers Inc., Aliso Viejo, California) (see Figure 1). The FDA initially approved the system for anterior capsulotomies and corneal incisions (August 2009) then added approval for lens fragmentation (April 2010).1 A search of the peer-reviewed literature indicates that it is the most-studied cataract laser to date.
System Overview
The LenSx femtosecond laser system uses an integrated Fourier-domain optical coherence tomography (OCT) imaging system.2,4–6 The OCT is used to plan the treatment based on measurements of the cornea, anterior and posterior lens capsule, and crystalline lens nucleus.5 The standard procedure sequence is capsulotomy, followed by lens fragmentation, then primary and secondary corneal incisions, and finally arcuate keratotomy or arcuate incisions if desired.1,2,7 Modifications to the procedure sequence can be made to accommodate different requirements: successful laser capsulotomy, lens fragmentation, and corneal incisions post-penetrating keratoplasty, and trabeculectomy have been reported.8 The preliminary docking procedure and the various steps above are described in detail in the following sections.
Docking Mechanism
An ideal docking mechanism limits eye movements and corneal folds to ensure appropriate eye stability and efficient supply of laser energy.9 This must be achieved while minimizing trauma to the eye and limiting intraocular pressure (IOP) rise, to reduce the potential for postsurgical complications.9 The latest version of the LenSx system uses a proprietary SoftFit™ interface: a 13 mm single piece curved contact lens and suction system to applanate the cornea.4,7,10–12 This interface is sterile, disposable, and mounted onto the objective (see Figure 2).1 The force applied by the objective is controlled by a spring-loaded objective lens.1 When the surgeon observes proper applanation as determined through the live video image, suction is applied.1 Suction is lower than in previous iterations of the interface, with an average IOP rise of about 16 mm Hg.
It is important that a docking system be effective and reliable, to reduce the number of docking attempts and the number of ‘suction breaks’, which would require additional manipulation of the eye to rectify. Technologic
advances, refinement of the procedure, and surgeon experience have significantly reduced the likelihood of problems with docking. In a recent study, the mean number of docking attempts and suction breaks for an experienced surgeon with the LenSx system were 1.05 attempts per success and 1.31 % suction breaks.8 Several risk factors for suction breaks were identified: young age, narrow palpebral apertures, strong saccadic eye movements, and flat corneas.1,3 After applanating the cornea with the docking system, suction is applied and there is a transient rise in IOP. Average docking time to complete the capsulorhexis, lens fragmentation, and corneal incisions is less than a minute.13 The low and transient rise in IOP is not associated with any changes in the macula in the immediate postoperative period. Early postoperative macular changes were reportedly higher in a control group undergoing manual phacoemulsification than in a test group undergoing femtosecond laser-assisted cataract surgery.14
Femtosecond Laser Planning
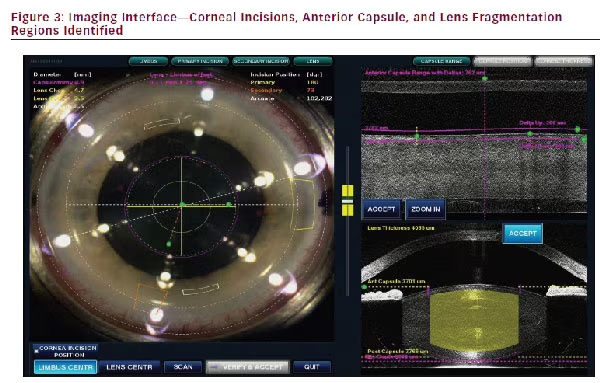
Once docked, the surgeon can view the cornea and lens in the video imaging system, and plan the location and size of the capsulorhexis, primary or side port incisions, as well as relaxing incisions if they are being made. The OCT image locates the anterior lens capsule, the lens nucleus, and the cornea in three dimensions. Single or multiplane clear corneal incisions (CCIs) can be planned. The volume of lens fragmentation can also be specified, with the surgeon using the imaging data to avoid laser fragmentation energy reaching the anterior or posterior capsule. Figure 3 shows the video interface.
Capsulotomy
The ideal capsulotomy is generally considered a circle of a precise size, well centered, and with no tags or tears. This increases the likelihood
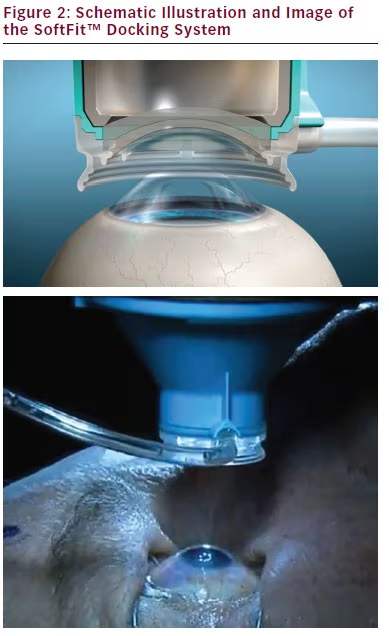
Figure 1: LenSx® Femtosecond Laser System Figure 2: Schematic Illustration and Image of the SoftFit™ Docking System of a uniform anterior capsule overlap on the IOL being implanted. Imaging studies have demonstrated the uniformity of laser-created capsulotomies. They have been shown to be well centered, more regularly shaped, with a more precise diameter, and better circularity than manual capsulotomies.5,13,15,16 This uniformity appears to have a positive effect on the strength of the remaining capsular structure, as demonstrated ex vivo in porcine eyes.13 Laser capsulotomies are generally free-floating and complete, with one study reporting 96 % of capsulotomies in that state.8
The laser-created capsulorhexis can be precisely positioned which increases the degree of IOL overlap.5 The percentage of capsule overlap with capsulotomies made with the LenSx femtosecond laser system has been demonstrated to be significantly higher than for manual capsulotomies (89 % vs 72 %, respectively).15,16 A study also demonstrated that when implanting a single-optic accommodating IOL, a 5.5 mm capsulotomy size resulted in less IOL tilt than a 6.0 mm capsulotomy size.17 The higher uniformity and better predictability of laser-created capsulotomies is expected to have a positive effect on IOL position

(particularly with regard to tilt and decentration) and anterior capsular forces on the IOL are likely to be more balanced. Studies have demonstrated that a laser capsulorhexis can be created even when conditions are not ideal, such as in traumatic cataract cases, Figure 3: Imaging Interface—Corneal Incisions, Anterior Capsule, and Lens Fragmentation Regions Identified or where pre-existing radial tears or anterior capsular lacerations exist.18 These situations can be problematic with manual capsulotomy because the tangential shearing forces from the forceps are affected by changes in the regularity of the anterior capsule, while the laser capsulotomy is not.
Complications have been reported, including small anterior capsular tags, anterior radial tears, posterior capsular rupture, and four cases (2 %) of dropped nuclei.1 However, complication rates have been demonstrably lower as a result of three factors: hardware and software improvements in the laser systems, procedural improvements in technique, and surgical experience. One study of 1,500 eyes reported overall capsule (anterior and posterior) complication rates of 7.5 % in the first 200 eyes with the LenSx system, dropping to 0.62 % in the subsequent 1,300 eyes.8 The study authors state that their complication rate with laser cataract surgery (LCS) is now similar to the best-reported complication rates in the literature for manual cataract surgery (MCS).
Lens Fragmentation
The lens liquefaction or fragmentation treatment algorithms for the LenSx laser system are customizable based on the surgeon’s preference; they are preprogrammed before the start of the procedure.1,19 It has been recommended to use liquefaction, a treatment pattern of concentric rings, in cataract grades up to 2.0.3 For cataract grades 1.5 up to 4.0, lens fragmentation is recommended.3 Lens fragmentation includes three available treatment patterns: cross (two perpendicular cuts), cake (six to eight cuts), or hybrid (concentric cuts with cake pattern).3 A hybrid pattern is shown in Figure 4; new lens fragmentation patterns are being developed and tested. The lack of transparency in a completely white traumatic cataract can interfere with the ability to perform laser treatment of the nucleus, but less-dense traumatic cataracts (e.g. grade 1–2) have been successfully treated.18
Corneal Incisions
Laser-created CCIs have been demonstrated to be extremely precise in both width and height,5 with good architectural stability.18 The LenSx system allows for single plane or multiplane incisions, with the dimensions selected by the surgeon in the planning stage of the procedure while viewing a cross-section of the cornea. A small percentage of incisions have been reported to be hard to open because of their self-sealing nature, or due to their far peripheral placement.3 In these instances a Femto-incision Spatula is typically used to identify incision interface and assist in opening the incisions created with a femto laser.
The primary and side-port incisions are full-thickness incisions. Arcuate incisions to address corneal astigmatism are also possible. They are generally made in the steeper meridian at a depth up to 80 % of the peripheral corneal thickness.3 The location, depth, and shape of the incision can be dictated by the surgeon. A number of algorithms have been developed for femto-laser incisions to correct astigmatism. One feature of a laser arcuate incision that is not possible with a blade is to make the incision and leave it ‘closed’. The stroma must be manually separated to ‘open’ it. This may be performed at the time of surgery, or at a later date, to titrate the patient’s refractive result.3
Overall, the use of the LenSx femtosecond laser system demonstrably reduces the amount of phacoemulsification energy required for all cataract surgeries.13,20 This lower energy appears to have a positive effect on endothelial cell counts and corneal edema after surgery, but further research in this area is required.20
Clinical Results
The promise of femtosecond LCS is a precision that cannot be matched with manual techniques. The repeatability of the various steps above is expected to lead to less variability in the effective IOL position, and higher predictability of clinical outcomes. Considerable research is being conducted in this area.
Higher Order Aberrations
One study that measured higher order aberrations after cataract surgery found that there were fewer internal aberrations in the femtolaser group relative to a control.21 The point spread function (PSF) showed a betterquality retinal image in the laser-treated eyes than the manually treated eyes. There was more evidence of coma in the manually treated eyes. Coma contributes to degradation of the PSF and is often associated with tilt or decentration of one component of the eye’s optical system. The authors suggest the more uniform capsulorhexis from the femtosecond laser system may contribute to lower amounts of tilt and/or decentration, with correspondingly lower levels of coma (and hence internal aberrations) in the eye. Additional research in this area would appear warranted.
Intraocular Lens Position (Tilt and Decentration)
Aspheric intraocular lens (IOL) decentration >0.4 mm or IOL tilt >5° can compromise visual performance.22 One study has demonstrated significantly lower levels of decentration and tilt in a femtosecond LCS group relative to a manual control group.15 No eye with laser treatment exceeded the 0.4 mm and 5° values above, though some eyes in the manual group did. The increased tilt observed in the manual group was associated with reduced corrected distance
visual acuity
in some patients, presumably a function of the resultant coma that is not treatable with a spectacle prescription.22 Visual Acuity In routine cataract surgery, several studies have demonstrated no measurable difference in visual acuity between the laser and manual cataract surgeries.21,23 The challenge is that cataract surgery overall (manual or laser) is a successful surgery, so demonstrating statistically significant improvements in clinical outcomes requires a large number of patients. Differences have been measured in some populations. Patients undergoing combined cataract surgery and vitrectomy cases exceeded their expected visual outcomes.24 Also, a greater percentage of eyes had 20/25 or better uncorrected distance visual acuity in the laser group than the manual group in a series of patients implanted with the AcrySof ReSTOR +3.00D IOL.23 It may be that the sensitivity to tilt and decentration is higher in this group where an advanced technology IOL is being implanted. Again, additional studies with large numbers of patients are warranted. The value of the laser technology may be in a reduction of the standard deviation of results, and this difference may be a bit more difficult to quantify than just a difference in the average postoperative acuity achieved.
Intraocular Lens Power Calculations Errors
Effective lens position is a critical element in the accuracy of IOL power calculations. Femtosecond laser systems are expected to improve the predictability of lens position based on a more uniform coverage of the IOL by the anterior capsule, potentially reducing asymmetric forces that may decenter, tilt, or move a lens in the z-direction. One study has demonstrated that IOL calculation errors were significantly lower in a laser group relative to a manual control (~0.12D).25 The improvement was greatest for eyes with short or long axial lengths, but the number of eyes in these extreme groups makes any definitive conclusion difficult.25 Again, more research in this area is warranted, and is underway.
A final comment is that femtosecond cataract surgery is evolving at a rapid pace. Numerous software and hardware refinements and procedural improvements have been made since the articles discussed here were published. Starting surgeons now are likely to achieve better results, in a shorter period of time, than those achieved by the clinical researchers and early adopters whose data are reported here.
Conclusion
The clinical data presented here suggest that even in its early adoption phase the use of femtosecond laser systems for cataract surgery provides predictability and clinical results that match the best manual phacoemulsification surgery. The precision of the capsulorhexis and corneal incisions contribute to lower variability in IOL position while laser fragmentation reduces the energy required for lens removal. Future advances in the technology are likely to raise the bar further. The demonstrated clinical impact of these improvements is likely to determine whether LCS becomes the new standard of care.